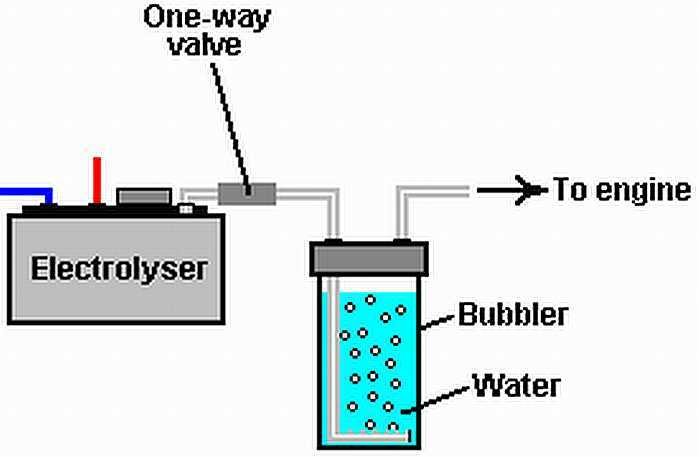
The method of decomposition of water into hydrogen outline, the manufacturing process is as follows; the use of this hydrogen element is intended to reduce the carbon dioxide (CO2) produced from the atmosphere, then deposited into biogas or collected using gas waste treatment.
Further carbon monoxide and hydrogen produced then synthesized into fuel with high purity by using the Fischer-Tropsch process. Excess heat from this process is then used to create more steam, Sunfire claims that this process has increased efficiency by up to 70 percent. One application of fuel to produce hydrogen, which converts water (H20) into hydrogen gas (H2) by electrolysis by using HHO Generator
There are at least three common methods that are currently used to decompose water into hydrogen, namely:
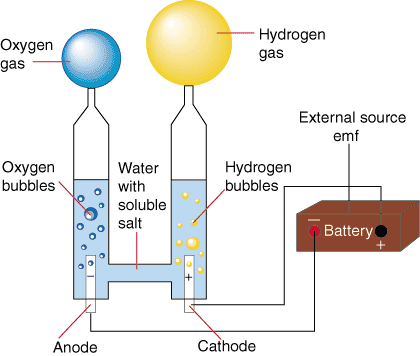
Electrolysis
In the electrolysis process, we pass electric current (read: give electric energy) to water so that water decomposes into hydrogen gas in cathode (negative pole) and oxygen gas in anode (positive pole). The resulting hydrogen gas can be channeled into the vehicle combustion system. Because water is not a good conductor of electricity then we usually add salt or acid or base in order to become electrolytic. Several studies have shown that water can conduct electricity in certain radio waves. We can easily do this at home using a hp or battery adapter where both cords are dipped in brine. Among all the methods of decomposition of water, electrocrolisis is the easiest method and most likely to be made portable.
Water as a renewable resource can be empowered using HHO GENERATOR. HHO GENERATOR in conducting electrolysis that converts water (H20) into hydrogen (H2). In this discussion, we will discuss the process of electrolyzing water (H20) into hydrogen (H2) with HHO GENERATOR device.
It has been more than 80 years of electrolysis process is used commercially, as well as gold plating, silver and so forth. The principle of electrolysis in ordinary water is to break the chemical bond of water (H2O) to H2 and O2, required direct DC voltage (DC), which is flowed through a stainless plate plate.
On the plate plate Kathoda charged (-) will occur / accumulated H2 gas and on the plate part of the charged Anode (+) will accumulate O2 gas, the two forms of gas will come out together so that the gas is called HHO gas. In this electrolysis process required electrolytes such as KOH, NaOH or Backing soda etc. Electrolytes are useful for channeling electric current in the water. If the H2 gas is used for combustion then the mixed O2 gas does not make the combustion obstacles because each combustion requires O2, even HHO has an advantage, without O2 from the outside can be ignited or burned.
HHO GENERATOR is a water electrolysis device that produces hydrogen (H2) stored in water (H2O), so water (H2O) can be separated into H2 (hydrogen) and O2 (oxygen). The process of electrolysis of water is also known as alkaline electrolysis, because for this electrolysis process is needed a catalyst solution is an alkaline solution (such as KOH, NaOH, Baking Soda and so on).
The electrolysis process uses 2 condenser plates, one plate is used for the Kathoda electrode (-) and the other for the anode (+) electrodes, both condenser plates are immersed in aqueous liquid mixed with KOH, if Kathoda and Anode are given an electric voltage it will occur at the electrode kathoda will be compressed H2 gas and the O2 compressed anode electrode, the result of the split can be used H2 gas to add or replace some of the fuel used for motor vehicles, thus reducing the fuel consumption itself automatically .
Wet electrolysis is commonly used electrolysis until now, by inserting both condenser plate into distilled water or RO water (Reverse Osmosis = pure water), if both electrode is given electric voltage hence will happened separation process H2 and O2, then result H2 needs to be calculated and in-data from the experimental results, otherwise it is necessary to examine the variables that affect the production of H2, so it can be concluded the most efficient use of electrolysis & amp; effective. Dry electrolyze is a new development which is usually called Dry-cell, in this process the condenser plate is not immersed into the solution but the plate is located outside and the solution inside the plate. In hot dry cell process
Electrolysis process formula:
At the electrode Kathoda electrons (e-), so the chemical reaction occurs as follows:
Kathode (Reduction):
2H + (liquid) + 2e- → H2 (gas).
While on the Anode electrode, there is oxidation process where the release of electrons moving towards the electrode kathoda, chemical reactions on the Anode as follows Anode (oxidation):
2H 2O (l) → O2 (gas) + 4H + (liquid) + 4e-; (l = Solution).
The chemical reaction of the balancing, water reaction with the basic solution as follows;
Coded (reduction): 2H 2O (l) + 2e- → H2 (gas) + 2OH- (liquid)
Anode (oxidation): 4OH- (liquid) → O2 (gas) + 2H 2O (l) + 4e-.
Reaction reaction of H2 and O2;
Overall reaction:
2H 2O (l) → 2H2 (gas) + O2 (gas).
From the equation of the chemical reaction occurring, the amount of H2 volume of gas of magnitude or volume is 2 X more than the amount of O2 gas volume.
Water electrolysis will produce HHO gas:
1 Liter Water = 1.750 Liter Gas
HHO gas form that happens there are 14 kinds
Flame Speed 1,300 Mtr / Second in Vacuum condition (P abs = - 820 mmHg)
Self Ignite: T = 550 C at P = 15 Psi
Reaction: Electrolysis of KOH solution in water:
Cathode: [2H2O (l) + 2e → 2OH- (aq) + H2 (g)] x 2
Anode: 4OH- (aq) → 2H2O (l) + O2 (g) + 4e +
2H2O (l) → 2 H2 (g) + O2 (g)
Reaction: Electrolysis of Na2CO3 solution in water:
Cathode: [2H2O (l) + 2e → 2OH- (aq) + H2 (g)] x 2
Anode: 2H2O (l) → 4H + (aq) + O2 (g) + 4e +
2H2O (l) → 2 H2 (g) + O2 (g)
In the electrolysis of a solution containing the IA (Na +, K +) ions, the ions are not reduced to the cathode but the water that is reduced by the reduction of water potential is greater than the reduction potential of Sodium or Potassium ions (Eo H2O / H2 = 0.83 volts and Eo Na + / Na = - 2.71 volts).
In the application of electrode used is stainless steell which can be categorized as inert electrode, from experiments we do on some motor vehicles, for 1000 CC cars with speed 50-60 km / h with fuel consumption (besin) 1 liter can travel 12 km , so it takes 12/60 hours = 12 minutes. If the calorific count is generated on the perfect combustion of 1 liter of gasoline (octane) by reaction: Ramsden E.N (2000: 499).
C8H18 (g) + 25/2 O2 (g) → 8 CO2 (g) + 9H2O (g) rHo = - 5510 kJ / mol
This reaction takes place in the combustion chamber, where the fuel oil has a boiling point of 150oC and will be vapor in the combustion chamber of the machine.
Mass 1 mol C8H18 = (8.12 + 18. 1) gram (Ar C = 12 and H = 1) = 114 grams
To burn 1 mol of C8H18 or 114 grams of C8H18 freed calor = 5510 kJ.
Gasoline mass = 0.77 kg / L so the mass of 1 L of gasoline = 770 grams, so the resulting heat = 770/114 x 5510 kJ = 37216.66 kJ
So the heat produced at burning 1 gram of gasoline = 37216.66 kJ / 770 gram = 48,333 kJ
At burning gasoline octane exhaust emissions still contain 5% CO gas.
In the assembly of electrolysis devices mounted on the car a diode with a strong Ampere current is used in the same time 12 minutes of hydrogen gas produced as follows:
Mass H2 = ME H2. i. t / 96500 gram = 1. 25. 12. 60/96500 gram = 0.186528 gram
Perfect burning of H2 gas according to reaction:
H2 (g) + ½ O2 (g) → H2O (g) rHo = -241,82 kJ / mol
At the burning of 1 mole or 2 grams of hydrogen gas produced heat = 241,82 kJ
For perfect combustion 1 gram of hydrogen gas produced heat = 120.91 kJ
For burning 0.186528 grams released heat = 0.186528 / 2 x 241,82 kJ = 22,5531 kJ
While the oxygen gas produced from the same electrolysis process:
Mass O2 = ME O2. i. t / 96500 grams = 32/4. 25. 12. 60/96500 grams = 1.49223 grams
The volume of O2 gas produced when measured at 25 ° C and 1 atm pressure is PV = nRT
or V = n RT / P
V gas O2 = 1.49223 / 32. 0.082056872. 298/1 Liter = 1,14027 Liter
THE FACTS ABOUT HYDROGEN
- Hydrogen has a burning speed of 3,600 x faster than gasoline. Therefore the process of explosion in the combustion chamber is faster so that the motor is more responsive.
- If it explodes in the combustion chamber, then the hydrogen produces heat far below the gasoline. So the engine room temperature is cooler than gasoline burning.
- The hydrogen explosion is implosive, not explosive. This means that only produce power with low heat. Remember, the explosion of Hiroshima and Nagasaki bombs. Both use hydrogen bombs and their damage effects are remarkable due to the energy released from the explosion.
- If using hydrogen, then the condition of the combustion chamber will be cleaner. That's because the nature of this gas is very easy to bind carbon. Well the pile of dirt in the combustion chamber was composed of a pile of carbon. If tied up by hydrogen in burning over time will be lost and the result is clean.
- Until now there is no pure combustion of hydrogen. That means gas is still needed. "Theoretically it can, but it requires the requirement of a stronger engine component or more than the condition of the engine innards at present.

Thermal Decomposition
Decomposition by panans is commonly used to aid the decomposition reaction. As the compound heats up, the atom moves harder, and this movement can break the chemical bond. For example, if the calcium carbonate (CaCO3) is very hot, it will break down into calcium oxide (CaO) and carbon dioxide (CO2).
The temperature required to decompose the compound depends on the bonding strength that keeps it together. In this example, calcium carbonate loses carbon atoms and two oxygen atoms as CO2, but calcium holds on to one oxygen atom because of the very strong calcium-oxygen bonds and can not be broken by warming with ordinary temperatures.
The more reactive elements tend to form stronger bonds and, therefore, are more difficult to separate from their compounds. In contrast to the above example, less reactive metal oxides, such as silver and mercury, can be decomposed by moderate heating, releasing oxygen and leaving pure metals. Highly reactive metals, such as sodium and potassium, can not be separated from their compounds by self-heating.
2.Electrolysis
In a liquid state, the element may be separated from a compound by applying an electric current directly in a process known as electrolysis. The current flows through the electrode, which is placed in a liquid. The negatively charged electrons flow into one electrode, known as the cathode, and out of the other, known as the anode. Because of this the cathode has a negative charge, and an anode, a positive charge. Ions perform movement in the liquid toward a reverse-charged electrode, allowing the current to flow.
An example is the decomposition of water into hydrogen and oxygen by electrolysis. Pure water is a very bad conductor, but the introduction of even very small amounts of ionic compounds, such as sodium sulfate, greatly increases the conductivity and allows electrolysis to take place. At the cathode, water (H2O) is divided into hydrogen gas (H2) and hydroxide ions (OH-), which are attracted to the positively charged anode. At the anode, water is divided into oxygen gas and hydrogen ions (H +), which are attracted to the cathode.
What is the usefulness of decomposition?
Thermal decomposition is used in the production of lime industry for the manufacture of cement and various other purposes. Electrolysis is used in the production of reactive metals. For example, sodium is produced by electrolysis of aqueous salt (sodium chloride). It also produces chlorine gas, which has many industrial uses, although most of the chlorine is produced by electrolysis of a salt solution in water. The decomposition reaction involving electrolysis is also used to make highly reactive fluorine elements, and as a "clean" way of producing hydrogen for fuel.
If in the electrolysis process we use electricity as an energy source, then in thermal decomposition we use heat to decompose water. Since water is one of the most stable substances it needs high temperatures (about 1200 - 1500 ^ {0} C) and catalysts (usually Pt and Ir).
2 H_ {2} O (gas) + heat \ rightarrow 2H_ {2} (gas) + O_ {2} (gas)
This technology is not too developed because it requires very high temperatures and the results obtained are not satisfactory so it is not possible to be portable in the vehicle. In addition, energy sources for heating are also a problem. If the energy source is more expensive it will be more efficient if directly used without having to convert water into hydrogen first. Natural gas for example will be more efficiently directly used as a fuel than used as a source of thermal decomposition of water.
If it requires hydrogen then natural gas can be converted directly into hydrogen through a steam-methane reforming process that gives better results and requires lower temperatures (around 700 - 1000 ^ {0} C).
CH_ {4} + H_ {2} O + hot \ rightarrow CO + 3H_ {2}
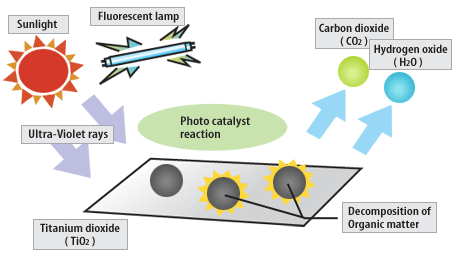
The term photocatalyst
The term photocatalyst is a combination of two words namely photographs and catalysis, so it can be interpreted as a process of a combination of photochemical reactions that require light and catalyst elements to accelerate the occurrence of chemical transformation. The transformation occurs on the surface of the catalyst whose catalyst is called a photocatalyst. Photocatalyst is one of AOPs (Advanced Oxidation Processes) method. Characteristics of AOPs are the formation of highly active free radicals, especially hydroxyl radicals (OH˙). Materials that can be photocatalyst is a semiconductor capable of adsorbing photons.
The photocatalyst process is widely applied for the removal or degradation of liquid pollutants into more environmentally friendly compounds, eg for phenol processing. A technology based on semiconductor photocatalyst irradiation such as titanium dioxide (TiO2), zinc oxide (ZnO) or cadmium sulfide (CdS) classified as heterogeneous photocatalyst [Hermann, 1999].
Heterogeneous photocatalysts are defined as catalysis processes in which one or more reaction steps take place in the presence of an electron-hole pair generated on the surface of a light-emitting semiconductor material at an appropriate energy level. The process can be done in various media, namely pure organic liquid phase and aqueous solution.
The overall process of heterogeneous catalysis reaction pests, both thermally activated (conventional catalysis) and light-activated (photocatalysts) are as follows
Transfer the reactant mass in the fluid phase (liquid or gas) to the surface of the catalyst.
Adsorption of reactants to the surface of the catalyst.
reactions in the adsorbed phase.
Desorption of the product from the surface.
Transfer of product (mass transfer) from inter-surface area (interphase).
Photocatalysis Mechanism
The phenomenon of photocatalysis begins with photoexcitation, as a result of an ultraviolet light on a semiconductor limb having greater energy than its semiconductor band, thus transferring electrons from the valence band to the conduction band while generating holes (h +) in the valence band. Thus, the process of photoexcation will produce electrons in the conduction band and holes on the valence band. The reaction that occurs for this phenomenon is
Semiconductors + hv (ecb- + hvb +) (2.1)
Furthermore, the electron-hole pairs formed will recombine within the particles (line B), and recombine on the surface of the particles (path A), but some will not recombine directly onto the surface of the particles. The recombination reaction of the h + / e pair is written as follows
Semiconductor (ecb- + hvb +) Semiconductors + heat
The electrons that arrive at the surface of the particle (line C) will donate itself to the adsorbed molecule on the surface where the molecule will undergo reduction resulting in the anion radical, A- (oxidator), whereas the hole up to the surface (line D) will draw the electrons from the molecule there is a surface so that molecules will experience oxidation. The adsorbed molecule is electron donor so that the hole capture result will produce the cation radical, D + (reductant). The reaction can be shown as follows
D (ads) + h + D + (ads) (2.3)
A (ads) + e- A- (ads) (2.4)
An adsorbed electron donor (reductant) can be oxidized by transfer of electrons to a hole above the surface and hole capture will produce an adult cation, D + (equation 2.3). however, the acceptor electron acceptor (oxidator) can be reduced by receiving an electron from the surface so that electron capture will produce anion radical, A- (equation 2.4).
The recombination reaction between electrons and holes can be indicated by the following equation:
e- + h + N + E (2.5)
where N is a neutral semiconductor material and E is energy released under UV light or semiconductor heat [Litter, 1999].
Semiconductor Catalyst
Semiconductors are materials that have an empty energy region (void energy region) called band gap (band gap) that is between the conductor and the insulator. Many types of semiconducting materials are commercially available but few are suitable for use as photocatalysts in decomposing various organic pollutants. The required criterion of semiconductor material as a catalyst is
- Being photoactive
- Able to use visible light or ultraviolet close
- Inert biologically and chemically
- Photostable (stable to light)
- Cheap and easy to get
- Insoluble in reaction
The semiconductor catalyst for the photocatalysis process consists of oxide and sulphide types. Semiconductor catalysts include oxide types eg TiO2, Fe2O3, ZnO, SnO2, and WO3, while those belonging to sulfide species for example CdS, CuS, and ZnS
The semiconductor material has a bandgap energy, the empty area extending from the top of the filled valence band (Filled Valency Band) to the base of the empty valency band (Vacant Conduction Band), sufficient to be excited by ultraviolet (UV) or visible light, and the reduction potential of the valance band (vb) and the conduction band (cb), can produce a series of oxidation and reduction reactions. The magnitude of the energy gap between the valence band and the conduction band will determine the thermal population level of the conduction band or, in other words, the electrical conductivity of the semiconductor. The piata gap defines the wavelength sensitivity of the corresponding semiconductor to radiation.
reference :
open-source
open-source
open-source
open-source
Posted on Utopian.io - Rewarding Open Source Contributors
Hello @aklilsteem, your contribution cannot be approved because it does not refer to or relate to an open-source repository. The repository which you have chosen here is for another project. Are you having problems understanding what open source project means ? Please kindly click here for a definition of "open-source." if you still have any further problems, please kindly contact us on Discord Channel. Thank you :)
[utopian-moderator]