The cell phones have revolutionalised the way we communicate. The technology of today has lots of uses. Some, like the cell phone, have got some people addicted to its use. Nowadays its hard to communicate with someone without the person occasionally looking at the cellphone. This increased mobility of phones is partly possible courtesy of batteries. Can you imagine the world without batteries? Your non-mechanical/electric clock will no longer keep time, the famous mobile phone will no longer be so mobile if we are constrained to be forever tethered to a wall; and the only mobility is the length offered by the power wires, we will have to find an alternative source of power to kick-start motors to ignite the engine, etc.
 Batteries.[CC0] ,
from Pixabay Commons
Batteries.[CC0] ,
from Pixabay Commons We love batteries for many reasons since it allows us easily store direct current until when we need it. Imagine a solar power system without batteries; you will only be limited to use during the sunny day. Emergency equipment like servers, hospital equipment, and businesses like the automated teller machines (ATM) that continually needs power can only easily have that happen without the existence of the battery. In the emergency theatres, lifesaving machines in hospitals hooked to the uninterruptible power supply (UPS) units- a backup power made whole via battery supply.
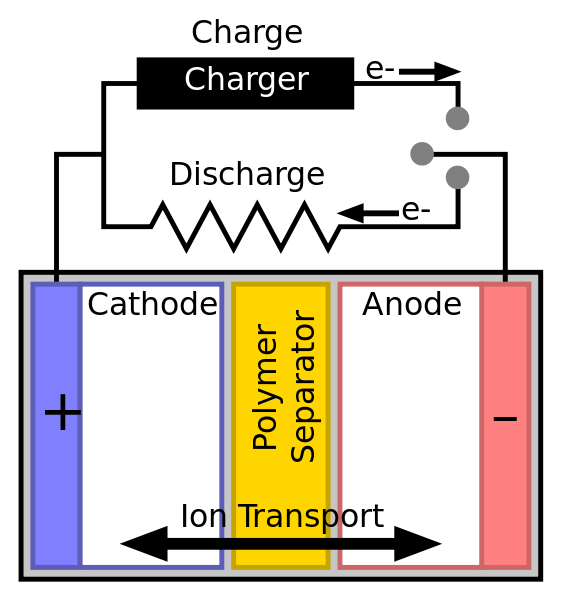 A simple battery diagram.By Tkarcher [CC BY-SA 3.0] ,
from Wikipedia Commons
A simple battery diagram.By Tkarcher [CC BY-SA 3.0] ,
from Wikipedia Commons The simplest battery is the cell, the main unit which is made up of three main parts. They are the two electrodes (one negatively charged anode and the other positively charged cathode) and the electrolytes. The electrolyte is the chemical which through a reaction produces electrons at the anode. The imbalance between the cathode and anode is what creates the electrical or potential difference.
But we have a gatekeeper known as the electrolyte. It prevents the electron-heavy anode from escaping to the cathode side of the battery.
But the gatekeeper opens the door when someone connects a load (e.g. bulb) between the cathode and anode. The electrons will then flow through the connection producing electricity that lights the lamp.
So when the electrons (negatively charged particles) stop flowing to the positively charged cathode, the battery will now be flat or "dead".
For rechargeable batteries, to reverse the process, the battery will "charge" and restore the charged state of the anode, ready for when next a load gets connected which opens up the electrochemical reaction which allows for the flow of electrons from anode until the electrons get depleted. The process is repeated up to a point the battery fails. That time, the anode is unable to hold "charge".
Each battery produced has a certain number of times it can be charged and discharged known as a cycle before it becomes incapable of sustaining charge, i.e. bad. For example, if a battery cycle is 300, it means it can be charged and discharged 300 times before it goes weak/bad or just utterly unable to retain charges.
Depth of Discharge
The battery's longevity is affected by the depth of discharge (DOD). The DOD is a measure of the percentage of the battery's capacity discharged during use. For instance, a 100AH battery that is fully charged (at 100%) has a DOD of 0%, but during use, if the battery lost 25AH of its capacity, it has lost 25% of its total capacity. We can say the DOD is 25%.
Some battery performance is dependent on the level of depth of discharge. Battery capacity can be said to handle 300cylces at 80% DOD of discharge. In other words, if the same battery is discharged up to 90%, the number of cycles will now be less than 300 cycles.
Tesla Powerwall batteries, from the Tesla Electric, uses nickel manganese cobalt oxide (NMC) cells which has 100% DOD capacity and up to 5000+ cycles, little wonder the Tesla Powerwall has a warranty of 10 years.
Ten years is a pretty long time for a battery to last, but if you think that was impressive wait till you meet the rover named Curiosity.
Curiosity Rover, a Battery Life to Envy
 Two spacecraft engineers stand to give scale to three generation of Mars rovers with Curiosity, the biggest one, standing 10 feet (3 meters) long. Notice the black projection on top of it, that is the battery powering it. By NASA [Public domain] ,
from Wikipedia Commons
Two spacecraft engineers stand to give scale to three generation of Mars rovers with Curiosity, the biggest one, standing 10 feet (3 meters) long. Notice the black projection on top of it, that is the battery powering it. By NASA [Public domain] ,
from Wikipedia Commons I know many wish their laptop battery or their cell phone battery could last a day. This desire is particularly strong in areas like some nations, like Nigeria, still grappling with the problem of electricity scarcity/unavailability; so that one full charge can last all day long. Well, if they are the Curiosity rover on a Mars mission, the wish for a battery that can last more than a day will be adequately fulfilled.
The battery in that rover is capable of powering the spacecraft for 14 years! That was some long time. Remember, unlike other rovers, Curiosity has no solar panel modules, the rover is as big as a car and weighs ten times bigger than its predecessors. The spacecraft engineers opted for this due to some disadvantages of relying on solar power such as decreased performance when dust covers the panel or during winter when there's the shortage of sun.
How did they do it?
The two words that made accounts for this longevity is nuclear and thermocouple.
The thermocouple is when two dissimilar metals have a different temperature between them, one hotter than the other, electricity will flow. We have a German physicist, Thomas Seebeck (1770–1831), to thank for his attempt at heating up dissimilar metals in 1821.
So as you can see, thermoelectric effect, when current is produced via heating up dissimilar metals on different temperature is possible via a thermocouple.
The Curiosity rover uses what the NASA scientist named as Multi-Mission Radioisotope Thermoelectric Generator, or simply MMRTG to run the rover.
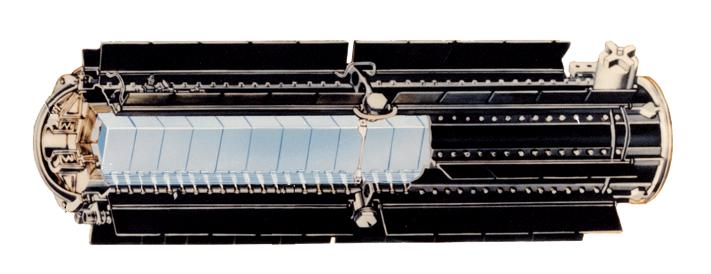 Sectional view of Radioisotope Thermoelectric Generators (RTGs) or space batteries. The U.S Department of Energy Office of Nuclear Energy, Science and Technology [Public domain] ,
from Wikipedia Commons
Sectional view of Radioisotope Thermoelectric Generators (RTGs) or space batteries. The U.S Department of Energy Office of Nuclear Energy, Science and Technology [Public domain] ,
from Wikipedia Commons The nuclear side of the space battery involves plutonium-238 which during its fission and eventually splitting of atoms produces heat which "cooks up" the thermocouple thereby producing thermoelectric properties in other to generate the electricity needed to power the big Mars explorer.
As you can see, the thermocouple is not a new technology. So also is the RTG technology in space exploration; it was first invented by Mound Laboratories in 1954 by the scientist duo John Birden and Ken Jordan. And first deployed in 1961 as a power source to a 175 pounds (approx 80kg) experimental satellite named Transit 4A.
Why don't we use RTG in our homes?
Though thermocouples are something common in everyday devices such as the refrigerators, air conditioners, and medical thermometers, you won't be seeing the use of an RTG in our home anytime soon. If we ignore the danger (toxicity and radioactive nature) of having a fissionable nuclear material such as plutonium at home, then the cost is crazily high. It costs about $4000 per gram, with terrorists and the likes interested in it, I do not expect the price to be coming down any time soon or even for it to be readily available for purchase.
Curiosity, powered presently, with about 5kg (5000grams) of plutonium-238 which makes the cost of the fuel to be around $20,000,000, a costing which is a conservative estimate as the process of making it is complex and takes a lot of resources.
References
If you write STEM (Science, Technology, Engineering, and Mathematics) related posts, consider joining #steemSTEM on discord here. If from Nigeria, there may be need to include the #stemng tag in your post. You can visit this blog by @stemng for more details. You can also check this blog post by @steemstem here and this guidelines here for help on how to be a member of @steemstem. Please also check this blog post from @steemstem on proper use of images devoid of copyright issues here
Would you like to delegate to the @steemstem? Here is a link below
50 SP | 100SP | 500SP | 1,000SP | 5,000SP | 10,000SP | 50,000SP

Hi @greenrun!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
I wish batteries will improve. They are still the blocker for many things :(
Me too. If there is a way to get the technology at par with other improvements in other areas, it'd make sense to have a battery that lasts for at least a week on single charge on our mobile devices.
It is especially important for electric cars. Concerning the phones, there were improvements. But the phones at the same time requires more power.
The more the functions, the more power hungry the phone becomes. You can imagine the difference between the first generation phone that can only make calls and text, compared to now a phone is virtually a mini PC capable of running a lot of functions.
Believe me, I cannot run what I do on a phone... I need hundreds of CPUs ;)
It's understandable when running a simulation/program which requires some special software and more resources due to the amount of computational power involved.
Exactly! :)
Batteries are really cool especially for our cellphones which is basically an extension of ourselves and the current lithium ion batteries are just so inadequate I came across a news article about a high school girl who made a supercapacitor to replace cellular batteries Source I'd like to know your take on it, it's been awhile since she made the discovery how come current cellphones aren't making use of the technology?
It is probably due to complexity and the behavior of supercapacitors. It may be a joy to charge at extreme short period, but the voltage of a supercapacitor is not as stable as the conventional battery during discharge. In order to make that stable requires some control circuit which will invariably increase the complexity, and of course cost, of the device.
Okay, thanks for your explanation I think I understand it better now although I believe they should be working on a better solution to our current batteries since it is really an issue for most people.
On another note, I'm currently soliciting votes for my latest post nd I was wondering if you wouldn't mind reading and probably upvoting it
You are welcome. I will check your post tomorrow; the voting power down for today. Keep steeming :)
Ok Sir. Thanks In Advance
I found myself memorizing what MMRTG stands for, like I was preparing for an exam.
Thanks for this information.
I hope you did end up memorising it.
Batteries are life saver.
14 years!! , that's really fascinating. It will be nice if we could have this in homes.
But with present limitations, I don't think it's anytime soon.
Interesting read
Yes the limitation is massive, we can only hope there's improvement in the technology we currently use.
14years of usage? That's really a long time. Ingenuity of humans will never cease.
I had wished it was possible to really use that at home especially in this part of the country, but the later part of the article explained it all.
Let's hope one day, further development will make it usable at homes, I guess we won't have to worry about no constant electricity supply.
This is a good one @greenrun
I have the same hope that either the battery technology improves, or we have improved devices that use much less power than we do now.
This is awesome! Battery is a device that is used to power electric devices, consisting of a set of electrically connected electrochemical or electrostatic cells. Without battery all devices will not function . Without battery the world in it's entirety will be on a standstill.
Great post Boss @greenrun
Battery is important. But I doubt if the world will be on standstill without it :)
Trust me @greenrun , the world will be on a standstill. For instance, the time clock uses battery both rechargeable and non rechargeable, if for some reason the battery is not available in the clock, how do we know our left from right? We will all be confused. Most machines uses battery, if the battery is not available how will they function? For instance without battery, a car cannot start, without battery, our phones and other gadgets cannot function, what about air planes, ships and many more.
Batteries are therefore important in our day to day activities. Without battery we will all go back to the stone age. Bringing the world to a standstill.
We really can't imagine the world without batteries, batteries have been the bank for our power save. We anticipate better advancement in developing high-end batteries that could last us a life-time (smile). A wonderful read. Thanks for sharing!
Thank you.
Is using smartphone while charging affects the longevity of battery?
If charging with the specified charger the answer is no. It only becomes an issue if while charging with the wrong charger, battery may swell up or even explode as a result of over charging.
Batteries are just too essential... Here in Nigeria we need batteries that can stay for up to 4days, I mean improvement is welcome but the risk that might be attached to it is my fear
All this 6000mah batteries are made for Nigerians I guess... Just saying
The capacity or size of a battery does not directly show the performance of such battery. I've seen the 2000mAh out perform a 4000mah battery. It all comes to efficiency of the processor and software. Some phones are just energy guzzlers, a 10,000mAh battery cannot even last for a full day of high usage.
This could really help the things to perform better over a longer term such a nice concept this is
I never understood electrodes and electrolytes in school... This makes sense
I'm glad I can help in that area. Thanks
Thanks for sharing this post....you have impacted with little knowledge.