INTRODUCTION
Petroleum industry is playing a vital role in the industrialization and meeting the basic needs of man through supplying energy for domestic, industrial, transport and as a raw material (feedstock) for fertilizers, synthetic rubber, polymers, explosives, dyes, paint, agrochemicals etc.
crude oil is refined to get different fractions in the refinery. Petroleum refining plays an important role in our lives.
 pixabay cc a refinery
pixabay cc a refinery
Petroleum consist mainly of hydrocarbon ( a series of molecules containing atoms of carbon and hydrogen). Petroleum is normally found at considerable depth beneath the earth surface.
Petroleum comprises of both crude oil and natural gas.
What is petroleum refining processes?
Petroleum refining process are chemical engineering processes and other facilities used in petroleum refineries to transform crude oil into useful products such as Liquified petroleum gas (LPG), Jet fuel, diesel, Kerosene (Atmospheric gas oil) etc.
History of petroleum refining
Petroleum and its derivatives has been known and used for almost 600 years and there is evidence of the use of asphalt in building more than 600 years ago.
Modern petroleum refining began in 1959 with the discovery of petroleum in Pennsylvania and subsequent commercialization.
Refining processes
1. Physical separation process
2. Chemical catalytic conversion process
3. Thermal chemical catalytic process
1. Physical separation process
This is divided into;
- Crude distillation
- Solvent extraction
- Solvent dewaxing
Crude distillation
This is the first separation process thats takes place in a refinery. It involves heating the crude oil in a distillation unit (fractionation column). Each of the fractions of the crude oil are separated according to their boiling ranges. The crude oil distillation unit is also called Atmospheric distillation unit because it operates slightly above the atmospheric pressure.
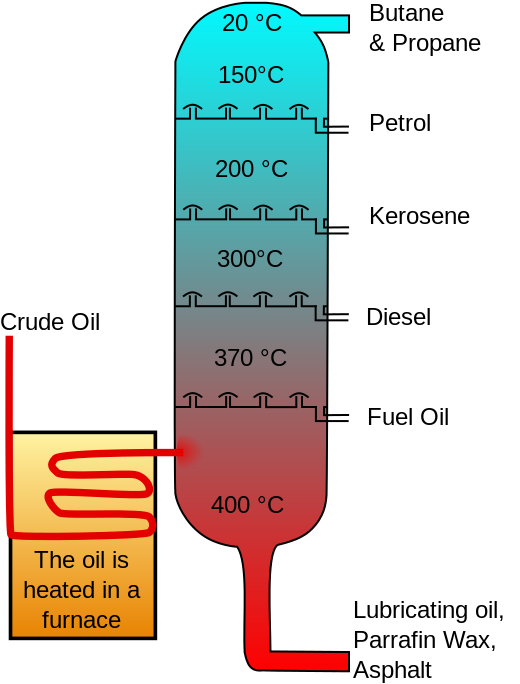 wikimedia cc schematic of adistillation column
wikimedia cc schematic of adistillation column
Solvent extraction
This is the process in which lubricating oil stock is treated by a solvent such as N-methyl pyrrolidole(NMP), which can dissolve the aromatic components in one
phase (extract) and the rest of the oil in another phase (raffinate) . The
solvent is removed from both phases and the raffinate is dewaxed.
Note: raffinate is the liquid which its impurities has been removed by solvent extraction.
Solvent dewaxing
The raffinate is dissolved in a solvent (methyl ethyl ketone) and the solution is gradually cooled, during which high molecular weight paraffin (wax) is crystallized, and the remaining solution is filtered. The extracted and dewaxed resulting oil is called ‘‘lube oil’’. In some modern refineries removal of aromatics and waxes is carried out by catalytic processes in ‘‘all hydrogenation process’’.
2. Chemical catalytic conversion processes
This involves the conversion of preheated petroleum hydrocarbon feedstock with a catalyst (catalyst contains phosphorus and transition metal modified silica rich zeolite)
The processes include;
- Catalytic reforming
- Hydrotreating
- Catalytic hydrocracking
- Alkylation
- Isomerization
Catalytic reforming
Catalytic reforming is the process of transforming C7-C10 hydrocarbon with low octane number to aromatic and iso-paraffin which have high octane number. It is also defined as the process of transforming low octane naphtha to to iso-paraffin and aromatics (Benzene). It is a highly endothermic process requiring large amount of energy. This reformate (product obtained) is used in gasoline formulation and as a feedstock for aromatic production (benzene).
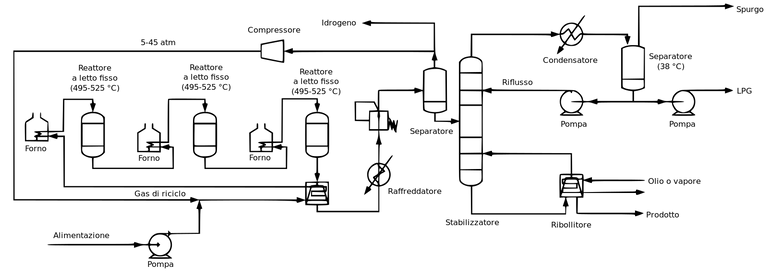 wikimedia cc catalytic reforming process
wikimedia cc catalytic reforming process
Hydrotreating
Hydrotreating is the process of removing impurities such as Sulphur, Nitrogen, aromatics from the petroleum with the use of catalyst, thus increasing the cetane number, density and smoke point. The catalyst is selected to suit the degree of hydrotreating and type of impurity. Catalysts, such as cobalt and molybdenum oxides on alumina matrix, are commonly used.
Catalytic hydrocracking
This is a chemical catalytic process used in petroleum refineries to transform high boiling boiling constituent hydrocarbon to a more valuable lower boiling product such as gasoline, Kerosene, jet fuel and diesel oil. The process occurs in a high hydrogen atmosphere at an elevated temperature.
Sulphur in the hydrocarbon is hydrogenated to hydrogen sulfide while the Nitrogen is hydrogenated to ammonia. These compound formed (hydrogen sulfide and ammonia) are easily removed from the petroleum.
Alkylation
This is a chemical refining process in which light and gaseous hydrocarbon are combined to produce high octane component of gasoline. The catalyst used is sulfuric acid or hydroflouric acid which are introduced in a reactor where they combine to form a mixture of heavier hydrocarbon. The liquid fraction fron Alkylation process is called Alkylate which consist mainly of isooctane (a compound which gives gasoline high anti knocking characteristics).
Isomerization
Isomerization of light naphtha is the process in which low octane number hydrocarbons (C4, C5, C6) are transformed to a branched product with the same carbon number. This process produces high octane number products. One main advantage of this process is to separate hexane (C6) before it
enters the reformer, thus preventing the formation of benzene whichproduces carcinogenic products on combustion with gasoline.
3. Thermal chemical conversion processes
These processes are considered as upgrading processes for vacuum residue (the substance obtained from vacuum distillation).
The processes include;
- Delayed coking
- Flexicoking
- Visbreaking
Delayed coking
This process is based on the thermal cracking of vacuum residue by carbon rejection forming coke and lighter products such as gases, gasoline and gas
oils. Three types of coke can be produced: sponge, shot and needle. The vacuum residue is heated in a furnace and flashed into large drums where coke is deposited on the walls of these drums, and the rest of the products are separated by distillation.
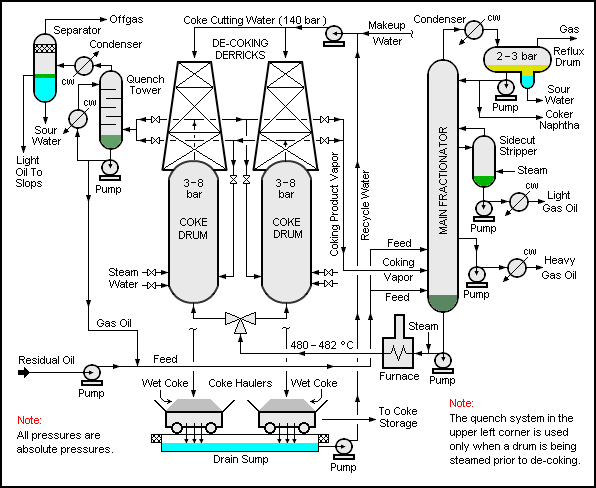 wikimedia cc delayed coking process mechanism
wikimedia cc delayed coking process mechanism
Flexicoking
In this thermal process, most of the coke is gasified into fuel gas using steam and air. The burning of coke by air will provide the heat required for thermal cracking. The products are gases, gasoline and gas oils with very little coke.
Visbreaking
This is a mild thermal cracking process used to break the high viscosity and pour points of vacuum residue to the level which can be used in further downstream processes. In this case, the residue is either broken in the furnace coil (coil visbreaking) or soaked in a reactor for a few minutes (soaker visbreaker). The products are gases, gasoline, gas oil and the
unconverted residue.
Thanks for reading through
REFERENCES
- Lecture note
- Fundamentals of Petroleum Engineering by MOHAMED A. FAH IM, TAHER A. ALSAHHAF, AND AMAL ELKILANI.
- wikipedia
- nptel
- Chemical engineering handbook
All images are Creative Commons or public domain, no copyright infringements have occurred.
.gif)
Thanks to @rocking-dave for the gif

Source
Plagiarism is the copying & pasting of others work without giving credit to the original author or artist. Plagiarized posts are considered spam.
Spam is discouraged by the community, and may result in action from the cheetah bot.
More information and tips on sharing content.
If you believe this comment is in error, please contact us in #disputes on Discord
Reminds me of CHM 101, and organic chemistry. I miss that. This article is informative, it refreshed my memory and I learnt a whole lot. Thanks for sharing
Nice post
I resteemed you.can we be friends