THE ATOM
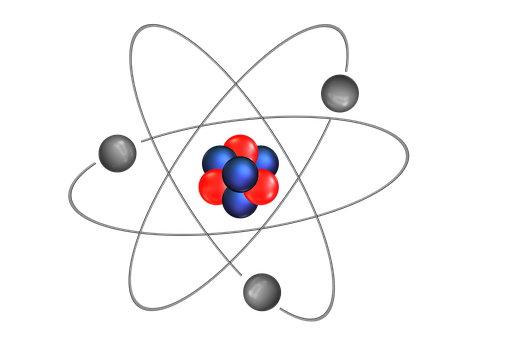
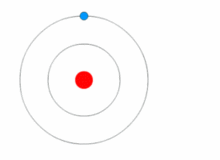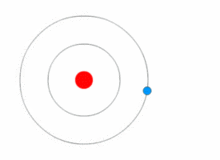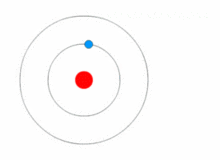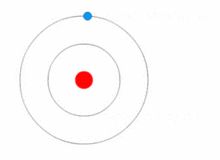
THE STRUCTURE OF AN ATOM
Atoms are present in everyday items that we see and use. An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. All matter is made up of tiny particles called atoms. Atoms themselves made up of three basic (sub-atomic) particles:
• Protons
• Neutrons
Protons have a positive charge, neutrons have no charge or uncharged and electrons have a negative charge. A collective name for particles found in the nucleus, for example protons and neutrons are called nucleons. An atom as a whole is usually uncharged or neutral because it has an equal number of protons and electrons.ELEMENTS

AN ELEMENT CALLED OGANESSON WHICH HAS AN ATOMIC WEIGHT OF 294
An element may be defined in one of two ways:
- An element is composed of identical atoms. If you could see the atoms that make up an element, for example gold you would notice that all the atoms are identical. Gold atoms each have 79 protons and 79 electrons. The element carbon would only be made up of carbon atoms containing 6 protons and 6 electrons.
- An element is the simplest form of matter. You cannot break down an element into simpler substances. For example the element carbon contains carbon (carbon atoms) and nothing else.
The number of protons determines the element for example if an element has 6 protons it can only be carbon. Over 100 elements have been discovered. When elements combine we get the different substances we see around us.
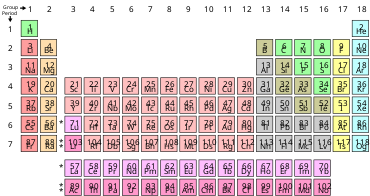
THE PERIODIC TABLE OF ELEMENTS
The periodic table used today is based on the one devised in 1869 by the Russian scientist Dmitri Mendeleev. Elements number 102 is named in his honour. The elements in the periodic table are arranged in order of increasing atomic number. Each little block contains information about an element.The vertical columns are called groups, two horizontal rows are called periods. The elements found in each group have similar chemical properties.
Metals on the left-hand side of the periodic table and non-metals are on the right-hand side of the periodic table. The elements in the middle of the table have properties of some metals and non-metals and are called semi-metals. The noble gases are a family of inert (do not react) or stable gases and form the last group in the periodic table.
COMPOUNDS
We are familiar with many elements for example oxygen, iron, aluminium and nitrogen. Most substances we see around us are not elements, for example clothes, plastic, paper. These substances are formed when elements combine with each other. Another substance that is a compound is water. Water is made when Hydrogen combines with Oxygen.
What is not a single element, but a combination of two elements. Hydrogen and oxygen are chemically joined (bonded) together to form water. Other examples of compounds sugar,(C, H and O), table salt (Na and CI) and carbon dioxide (C and O).
A compound can be separated into its Elements by chemical means only. It could be separated through heat or electrolysis (use of electricity). Water can be separated into its elements hydrogen and oxygen by electrolysis.
MOLECULES
An element is composed of tiny identical particles called atoms. If we could see the tiny particles that make up, water we would see a combination of hydrogen and oxygen atoms. A tiny particle which is a combination of two or more atoms is called a molecule. There are billions upon billions of molecules in a single drop of water.CHEMICAL FORMULAE OF COMPOUNDS
A chemical formula shows the chemical symbol of each element and the number of atoms of each element in a molecule of a compound.
In a chemical formula, if one atom of an element is present, the number one as a subscript (small number) is not shown. A chemical symbol itself means that only one atom is present.
NAMING COMPOUNDS
Many compounds are named according to the elements that they consist of. For example, sodium chloride is made up of sodium and chlorine.
Rule for naming compounds:
RULE 1: the -ide rule
When a metal reacts with a non-metal, the name of the compound formed takes on the name of the metal. The non-metal becomes the last part of the name and the end of it’s name is changed to -ide
When certain non-metals react with oxygen, the name of the compound will end with oxide, depending on the number of oxygen atoms in a molecule of the compound:
• MONOXIDE – when oxygen atoms combined with a non-metal
• DIOXIDE – when two oxygen atoms have combined with a non-metal
• TRIOXIDE- when three oxygen atoms have combined with a non-metal.
CHEMICAL REACTION
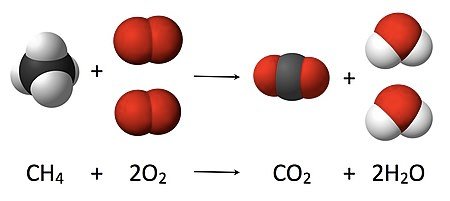
A CHEMICAL REACTION OF ELEMENTS
A chemical reaction takes place when two or more elements or compounds react together to form a new substance. The new substance has different properties when compared to the properties of the substances that react with each other. For example when sodium reacts with chlorine, sodium chloride or table salt is formed. In a chemical reaction energy is either released or absorbed.
THE END
References
1.https://pixabay.com/en/lithium-atom-isolated-atomic-2784853/
2.https://en.wikipedia.org/wiki/Periodic_table
3.https://pixabay.com/en/oganesson-chemistry-periodic-table-1740914/