Faraday’s laws of electrolysis
The factors affecting the quantities of matter liberated during the process of electrolysis were investigated by Faraday. 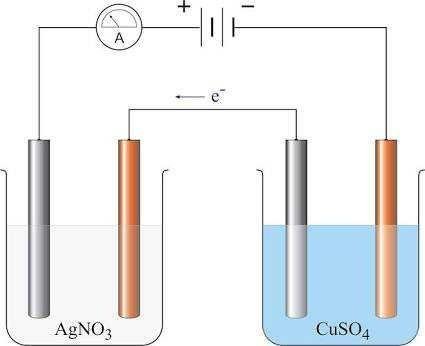
First Law :
The mass of a substance liberated at an electrode is
directly proportional to the charge passing through the electrolyte.
If an electric current I is passed through an electrolyte for a time t, the amount of charge (q) passed is I t. According to the law, mass of substance liberated (m) is
m α q or m = zIt
where Z is a constant for the substance being liberated called as electrochemical equivalent.
Its unit is kg C–1.
The electrochemical equivalent of a substance is defined as the mass of substance liberated in electrolysis when one coulomb charge is passed through the electrolyte.
Second Law :
The mass of a substance liberated at an electrode
by a given amount of charge is proportional to the chemical equivalent of the substance.
If E is the chemical equivalent of a substance, from the second law.
m α E
Source
There is reasonable evidence that this article has been spun, rewritten, or reworded. Repeatedly posting such content is considered spam.
Spam is discouraged by the community, and may result in action from the cheetah bot.
More information and tips on sharing content.
If you believe this comment is in error, please contact us in #disputes on Discord
fuck off