Hi to all the public of steemit, especially to the followers and lovers of science that make life in the scientific community of steemSTEM, I have been absent for several days from the network regarding publications, however, I try to keep myself informed of the growth and the dynamics of this great community, which every day seems to increase both in quantity and quality. For this post I will approach a basic topic related to the conception of the atom and the models that in principle gave shape to this imperceptible expression of matter.
Source (CC-BY-SA 3.0)
For the year 1910, it was certain that the atoms possessed electrons, this was manifested in the experiments carried out on the photoelectric effect, the dispersion of X-rays and others, such as the ratio e / m of the electron, the results of these works they established a magnitude Z, which indicates the number of electrons in the atom, and since the atoms are basically neutral, it is presumed that the number of negatively charged electrons must be equal to positive charge electrons. he knew that the mass of the electrons was very small with respect to the total mass of the atom, which logically indicates that the positive charge contained the greatest amount of mass.
All these data and considerations raised the problem of the distribution of electrons in the atom, mechanically speaking, so that led to the development of the famous atomic models built by Thomson, Rutherford and Bohr, in order to know the behavior of these particles in the atom.
Thomson Model
In a very summarized way in the atomic model of Thomson the electrons were considered as small particles located in "fixed" points within a continuous distribution of positive charge and spherical shape, in which given to the repulsions between loads, these must be located uniformly in the sphere. This model is known as "raisin cake" illustrated in Figure 1.
Modelo de Rutherford
Later in 1911 Rutherford through the dispersion of alpha particles, I can show that the positive charge is not distributed throughout the atom, but rather in a small region in the nucleus where almost all the The mass of the element is concentrated (figure 2), leaving the electrons orbiting around it.
Even when it was possible to advance in a more accurate perception of the atoms, there were strong doubts about how the electrons are distributed around the nucleus, and this is due to the fact that the Coulomb Force that exists between the particles does not allow us to conceive a disposition where the electrons do not fall to the nucleus, and if there was a shape such as the orbital trajectories of the planets around the sun, there was no explanation for the case of excited electrons, since in this state, acceleration causes a radiation electromagnetic that weakens the mechanical energy of the electron, allowing it to spiral down into the nucleus. Then, as you can see, there was no description of the problem of the stability of the atom.
Atomic model of Bohr
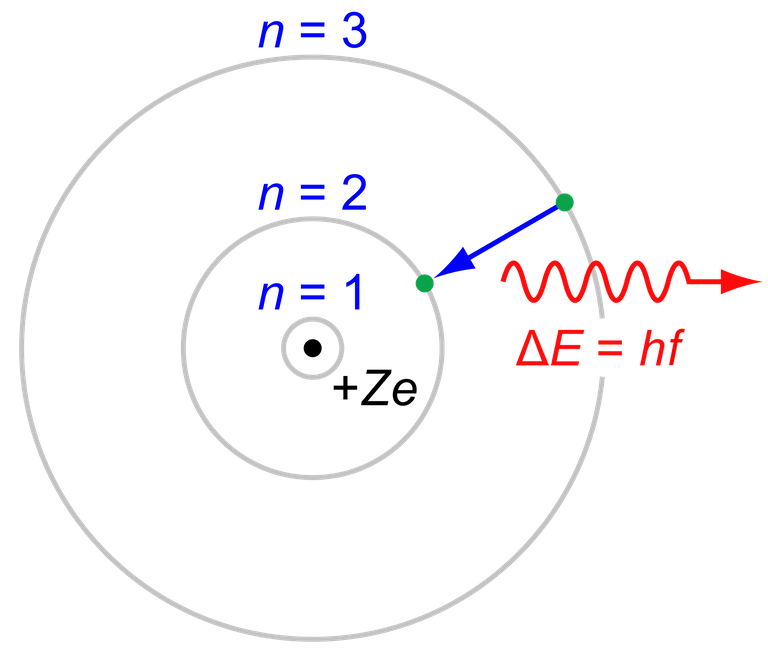
In 1913 Niels Bohr proposed his model that successfully achieved predicting the atomic spectrum of the most abundant element of nature [1]
The atomic model of Bohr, gives a quantitative and qualitative explanation of the behavior of atomic spectra, this is defined by the following postulates:
1.An electron in an atom moves in a circular orbit around the nucleus under the influence of the Coulomb attraction between the electron and the nucleus, subjecting itself to the laws of classical mechanics.
2.Instead of an infinity of orbits that is possible in classical mechanics, for an electron it is only possible to move in an orbit for which angular impulse. It is an integer multiple of the Planck constant divided by 2().
3.Even though the electron constantly accelerates when it moves in one of these allowed orbits, it does not radiate electromagnetic energy. Then, his total energy E remains constant.
4.Electromagnetic radiation is emitted if an electron, which initially moves in a total energy orbit, changes its motion discontinuously to move in an orbit of total energy
. The frequency of the emitted radiation is equal to the amount
divided by the Planck constant
.
[2]
The above corresponds to the quantitative explanation established by Borh for the structure and behavior of electrons in the atom, a curious mixture of the laws of classical mechanics can be noted with the innovation at that time of quantization of Planck energy, although seeing it well it does not sound so out of place to apply such concepts since we are talking about a quantum kingdom, very studied today. Now with respect to the quantitative form, it is easy to see this by developing the following example:
Consider an atom with a single electron orbiting its nucleus, that is, we will study the hydrogen atom the simplest element of the universe known so far, which consists of Z = 1. So by applying Newton's second law and the forces in the circular path of the electron, we have:
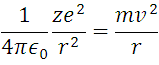
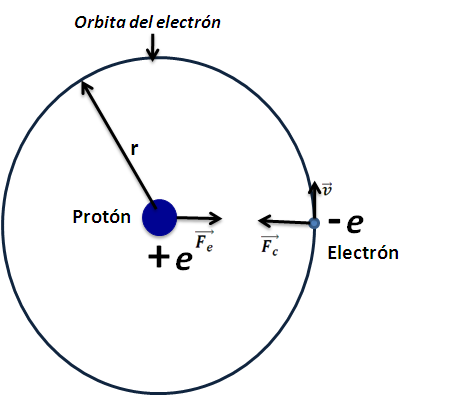
Coulomb force and circular motion
Where:
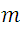 : the mass of the electron.
: the mass of the electron.
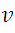 : the speed of the electron in its circular path.
: the speed of the electron in its circular path.
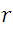 : the radius of the circular path.
: the radius of the circular path.
It is important to mention that (1) is an idealization of the phenomenon, like all modeling but with considerations of physical sense, given that the electron is under the influence of a centripetal force apart from the Coulomb Force, it is clear then that the acceleration Experience the electron is given by the expression that accompanies the mass in the right limb of the equation. Now applying the second postulate:
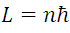
(2) represents the angular impulse of the electron according to Borh where n it can take whole solitary values as follows n = 1, 2, 3 ... And since it L is constant with a force acting in the radial direction we can write:
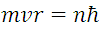
solving for 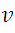 , and substituting in (1)
, and substituting in (1)
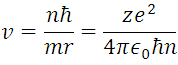
With n = 1, 2, 3, 4 ...
We take 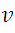 and substitute in (1), then we solve
and substitute in (1), then we solve 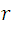 and we have:
and we have:
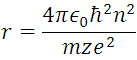
For n = 1, Z = 1 (hydrogen atom) and the constants already known, we can obtain 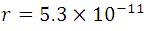 m, and
m, and 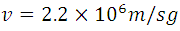 , this information represents the radius of Bohr for the hydrogen atom when the electron is in its ground state of energy, this it is appreciable in (5) where r are the circular orbits whose values are given by n which are in this case the allowed orbits. Now calculating the energy in these trajectories we apply the definition of potential, with the Coulomb Force shown in (1).
, this information represents the radius of Bohr for the hydrogen atom when the electron is in its ground state of energy, this it is appreciable in (5) where r are the circular orbits whose values are given by n which are in this case the allowed orbits. Now calculating the energy in these trajectories we apply the definition of potential, with the Coulomb Force shown in (1).
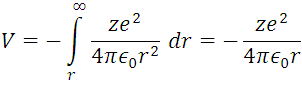
And for the kinetic energy also taken from (1)
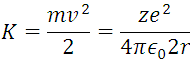
Therefore the energy of the electron is given by the sum of the 2 previous expressions:
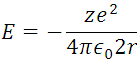
Taking into account (5) we have left:
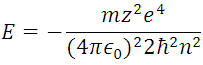
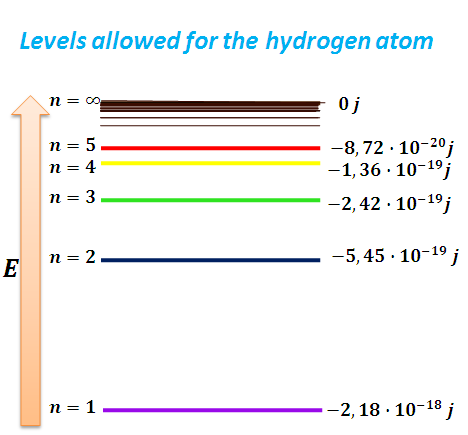
(6) indicates the energy that each possible level or orbit that can occupy the electro in the hydrogen atom whose values are represented in the following figure.
These values agree very well with the data obtained experimentally, as well as the frequency of the electromagnetic radiation given by:
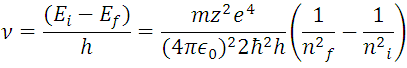
With (7) the value of the spectral lines for the hydrogen atom can be obtained in terms of frequency
obeying the fourth postulate of Bohr, and taking into account the relation 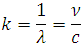 , rewriting (7) as follows:
, rewriting (7) as follows:
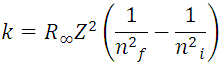
with:
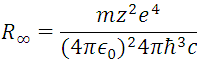
(8) represents the reciprocal of the wavelength of the electromagnetic radiation of all possible transitions of the electron at the energy levels of the hydrogen atom, with the proviso that ni > nf. In fact, this last expression contains the series of Balmer, Lyman, Paschen, Brackett and Pfund establishing the corresponding value of nf, for each interval, that is, the experimental data of the hydrogen atomic spectrum that founded the mentioned series, are deductible through of the Borh atomic model, which give the values of the spectral lines of hydrogen in all ranges, as shown in Figure 4.
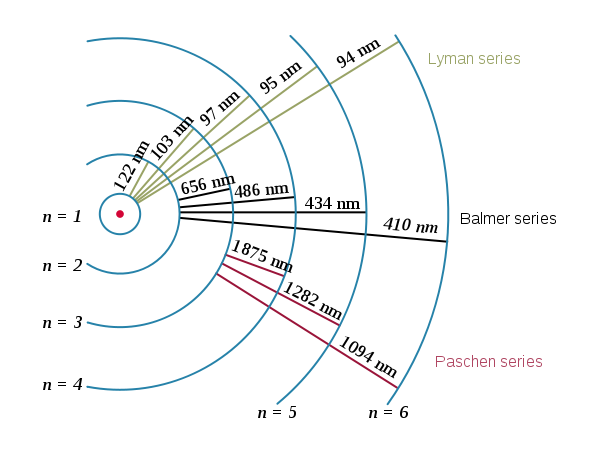
In addition it can be noted by the wavelength, that the radiation in the interval of the Paschen series, are less energetic than those of Lyman, given the jump difference in energy levels, ie a change of n = 6 to n = 1 emits a light more frequently, than from n = 4 to n = 3, and that is why these emissions are below (infrared) and above (ultraviolet) our visual range.
The above shows the correctness of this theory, although only limited to certain elements that meet the conditions proposed by Borh, represents a remarkable scientific breakthrough in the area of quantum physics, for me it is great and appropriate, simple and elegant of this method , which at that time was supported by the results of the research carried out by Planck and *Einstein, which clearly is a fundamental aid, I am impressed by the fact of having the vision in order to put together the pieces necessary to give shape to this successful theory .
I did really enjoy doing this little post about the most important and interesting advances of science in the field of Physics, I hope it will be useful or pleasant for you and I appreciate your attention and support. Greetings everyone
I recommend you visit the tag #steemstem, to read interesting articles of good quality regarding Science, Technology, Engineering and Mathematics
Elaborated by @joseg
References:
[2] Física cuántica. Eisberg- Resnick. Limusa Wiley. Modelo atomico de Bohr. pp.129-132.
Física Moderna. Virgilio Acosta. Clyde L. Covan. Graham. El modelo de Borh pp. 129-133.
The images that do not have a source are of own elaboration with the help of power point 2007
)
)
)

Congratulations @joseg! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
To support your work, I also upvoted your post!
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP