Hello to all my dear Steemians, this time I want to talk about a topic, which in my opinion, is very, very interesting, the engine of space rockets.
And it causes concern to imagine, the large amount of energy that must release this engine, to blow up such a heavy equipment as the space rocket, and that is not the only interrogating cause.
What material is burned? How is the motor made? anyway.
I invite you all to join me on this interesting topic.

First, the engine.
A rocket engine is a jet engine that generates thrust by expelling gases that come from the combustion chamber into the atmosphere. Rocket engines incorporate both fuels, which is usually kerosene or liquid hydrogen, and the oxidizer (oxygen in a gaseous or generally liquid state).
The rocket engine is the most powerful engine known and its weight/power ratio makes it the ideal engine to be used in spacecraft.
Reaction engine
It is a type of engine that discharges a jet of fluid at high speed to generate a thrust in accordance with the laws established by Newton. This generalized definition of the jet engine includes turbofans, rocket motors, ramjets, turbojets, and pulse reactors, but, in common usage, the term generally refers to a gas turbine used to produce a jet of gases for propulsion purposes.
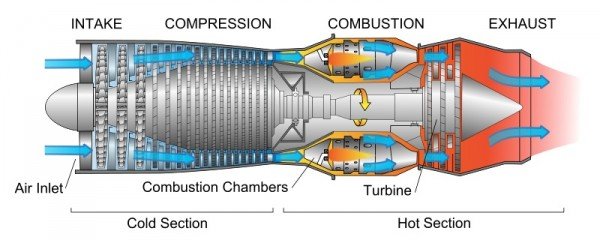
Types of Rocket Engine
-Solid propellant engines
A solid propellant is a type of fuel whose components are in solid form. Like the other propellants, the solid propellant is a source of thermochemical energy that is usually used in certain rockets as a means of propulsion. Unlike liquid propellants, once the combustion of the same has begun, the process can no longer be stopped until the thermochemical reaction has been completely completed.

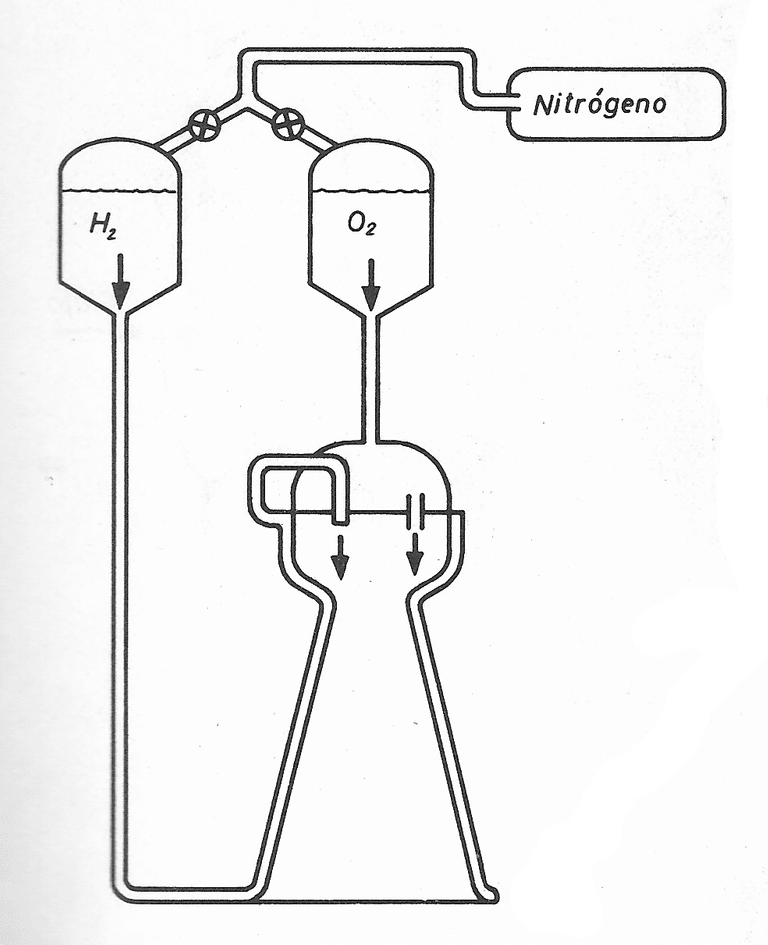
-Motors of liquid propellant
The different parameters: Specific Impulse, Calorific Power, Push, do not only depend on the thermodynamic and chemical characteristics of the propellant, but also on how it is burned in the combustion chamber. This interdependence of the Propergol - Impeller system leads us to consider briefly the mechanical units that allow a correct operation of the liquids.
The two fuels and fuel, stored in separate containers, are sent to the thrust generator by a power system; then they are injected into one of the extremities of the chamber, in the form of a mist, to be buried there. From these preliminary data, three sets of operations can be defined.
These are:
- The tank group.
- The feeding system, whose purpose is proportional to the thrust generator the quantities of propellant that are necessary at each moment.
- The push generator.
-Hybrid propellant engines
A hybrid rocket motor is the one that uses a liquid oxidant that in this case is hydrogen peroxide and a solid fuel that is generally a plastic.
The Hydrogen Peroxide Rockets are mono-propellant, this means that a single fuel is used for propulsion, in the case of Hydrogen Peroxide hybrid rockets, this is used not as a propellant but as a source of oxygen in the combustion since these rockets produce the thrust not by a single peroxide reaction but by a combustion.
This type of engine can have up to twice as much thrust as a mono-propellant of hydrogen peroxide using the same amount of peroxide in both cases.
The most common fuel in a hydrogen peroxide hybrid rocket is high-densityy polyethylene or polymethylmethacrylate, a very neat name but one that is vile Plexiglas or acrylic.
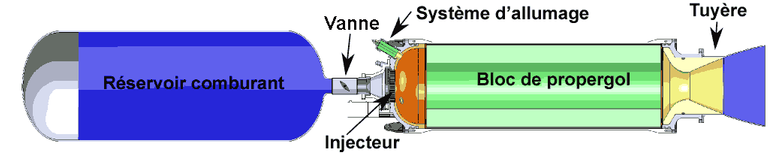
Basic principle of engine operation
The rocket engines produce the thrust by the expulsion at high speed of a fluid. This fluid is, almost always, 1 a gas generated by combustion inside a combustion chamber at high pressure (20-200 bar) of liquid or solid propellants, which consists of two components: oxidant and fuel.
The fluid outlet is passed through a supersonic propulsion nozzle which uses the heat energy of the gas to accelerate the exhaust at a very high velocity, and the reaction force to this pushes the engine in the opposite direction.
In rocket motors, high temperatures and pressures favor good performance, because it allows longer nozzles to be mounted in the engine, which provides higher exhaust speeds, as well as a better thermodynamic performance.
Second, the fuel
The airplanes that operate inside the atmosphere use the air considerably since they need it as support for their wings, they burn the fuel with their oxygen and, if they have propellers, they require air at a certain pressure so that they can "cling".
Since there are so few gas molecules above the Earth's atmosphere, conventional methods of propulsion are insufficient, while the lack of oxygen forces the aircraft to carry with them their supply of it, either in the form of the element itself, in a liquid state, or in the form of an oxidizing compound.
Several types of fuels and oxygen sources have been invented for the propulsion of rockets and other space vehicles, but the fundamental principle of all rocket propulsion is the same in all cases, that is, the principle of action and reaction of the dynamic.
When the fuel is burned, whether solid or liquid, it creates enormous quantities of hot gases, which are under great pressure, due to the small size of the combustion chamber. The gases that escape through the back of the engine provide the necessary thrust to propel it forward. Americans seem to prefer liquid propellants. Among the combinations that have been used successfully is hydrogen and liquid oxygen, which combine to produce steam. They also used liquid oxygen as an oxidant of fuels such as kerosene and ammonia.
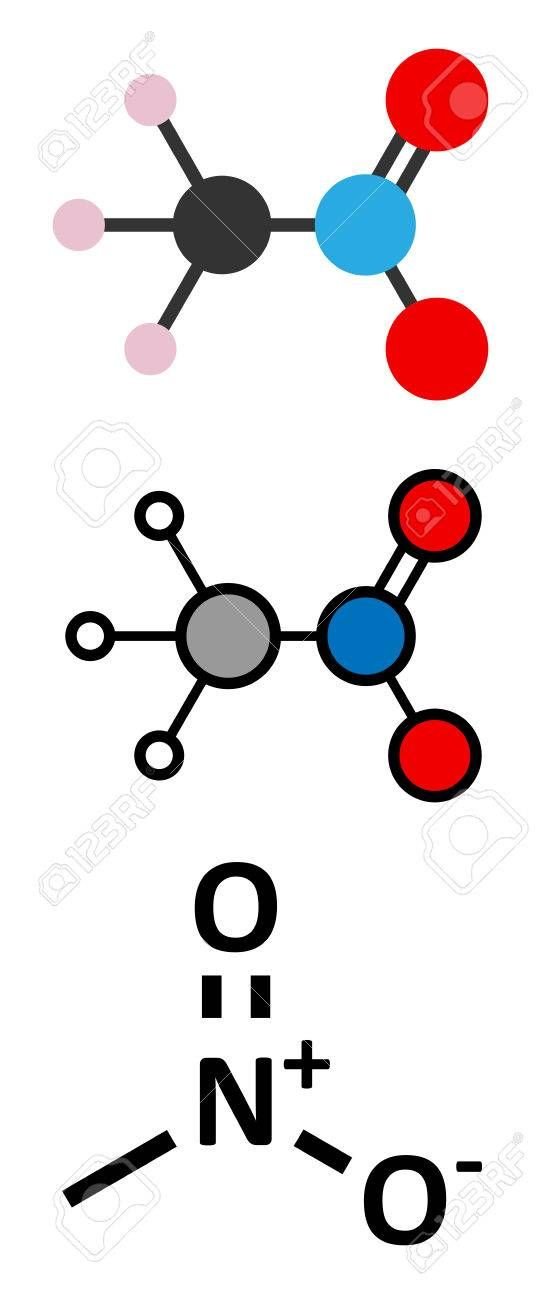
The combustion of kerosene produces steam and carbon dioxide. As an alternative, instead of oxygen, they have sometimes used hydrogen peroxide (hydrogen peroxide), which decomposes into oxygen and steam. In other rockets, the combination was dimethylhydrazinee, oxidized by nitric acid.
When liquid propellants are used, the fuel and oxidant must be kept in separate containers. The solid propellants, on the contrary, must be mixed before forming the "cakes" of compact fuel. The typical ingredients of one of these cakes can be: aluminum perchlorate (oxidizing agent), acrylic acid polybutadiene and aluminum powder (fuel), plus an agent that has the function of hardening the cake after it has been formed in mold.
The proportions of oxidizing agent and fuel are arranged so that there is always a slight excess of oxidizing agent. For this there are two reasons: if the amount of oxidizing agent is just enough for complete combustion, there is a serious risk of explosion, and, in addition, the pressure increase suffered by the excess oxidizing agent, due to the heat of combustion, It is added to the total pressure increase inside the combustion chamber of the rocket engine.
Diferent Source of propulsary energy
The maximum utilization of the available energy is obtained by the fuel of a rocket, when the exit velocity of the combustion gases equals the speed with which the rocket moves forward. It is therefore convenient to communicate the initial energy by some external means, such as a powerful motor, which provides the energy of movement, or kinetic, required for its speed to reach a value close to the output of the combustion gases.
The principle of action and reaction, on which the rocket propulsion is based, can be applied not only with conventional solid or liquid fuels, but could also think of energy obtainable in different ways. In fact, we know that electric charges, placed at a point in space where there is an electric field, experience forces of an electrical nature. In modern charged particle accelerators, high velocity values are obtained. This would be an appropriate method to obtain the necessary energy for the rocket drive.
The particles could be accelerated by powerful electric fields. Since matter is a hard concentration of energy, as it emerges from the theory of the relativity of modern physics, it is easy to see that a fuel like this would occupy little space, but instead, complex devices built by the most advanced technology would be indispensable.
Solid propellant rockets have been known for a long time, but they were only used again a few years ago. This was due to the simplicity of the system and the ease of storage. The research works with solid fuels are, at present, constant.
In the United States, a solid fuel with the consistency of rubber has been developed in the 1970s. It is the Thiokol, which is manufactured based on a derivative of liquid synthetic rubber, mixed with a solid oxidant. The potential yield of a fuel depends on the effectiveness of the oxidation.
An idea of the goodness of the fuel is given by the specific impulse. Using nitric acid as oxidant and aniline as fuel, a specific thrust of 221 is obtained, while using oxygen as oxidant and ethyl alcohol as fuel, a specific thrust of 242 is obtained.

Fluorine as oxidant and ammonia as fuel gives a specific thrust fuel mixture equal to 288. Ozone and hydrogen, as oxidant and fuel respectively, give a specific thrust mixture equal to 369.
Fluorine is one of the most effective oxidizing agents known. It is very probable, however, that the knowledge of some oxidant o the superior performance of the ozone type is kept secret. Fluorine gives good oxidant performance, especially with fluorite, but the cost of this mixture is currently very high. Ozone has a greater oxidant power than fluorine, but it has the disadvantage that in its pure state it shows a marked tendency to decompose explosively.

Trimethyl is an aluminum compound, fluid and extremely high flammable power. Its combustion is spontaneous when in contact with the air. Its application to rocket propulsion is not developed; but it can be a useful element for the future.

A source of energy hitherto practically unknown is in free radicals, which are nothing more than molecular fragments free of electrical charge, which are formed during an exothermic reaction. The upper regions of the Earth's atmosphere are a virtually inexhaustible source of these free radicals, which are caused by solar radiation. Free radicals are the result of a process in which they absorb energy. Then they can supply that energy for propulsion.
Recently, several experimental and research works have been carried out. The tendency of these works is to isolate free rad, calls to take advantage of them as fuels. The free radicals of water vapor, ammonia, hydrogen and nitrogen have been isolated.
The specific thrust of fuels based on free radicals is much higher than that of normal elements. Thus, for example, in the case of hydrogen, if a fuel with hydrogen is produced in the form of a free radical, a fuel is obtained whose specific thrust is five to six times higher than that of conventional fuels. In this way, a rocket, propelled with free radical-based fuels, will have a range of about thirty times that of the common type. However, these advantages of free radicals, in terms of energy concentration, have not left the theoretical field, because it is necessary to solve other very complicated problems, such as large and cheap production, storage, control and stability.
Using the ammonia radical with the liquid hydrogen, the range of the common rockets is raised to 7 or 8 times. Ionic propulsion is undoubtedly the most appropriate means to propel spacecraft. In this procedure, the appropriate ion source is cesium.
The ions of this element, accelerated in an incandescent surface of tungsten, and placed under the action of a potential of about 30,000 volts, can reach a speed of about 220,000 m / sec., Which is required for the specific impulse to be high .
Beryllium is an element that has a high calorific value per unit weight, but is very toxic, and is found in nature in relatively small amounts. On the other hand, lithium, which is an alkali metal, and boron, metalloid, allow combinations with hydrogen, called hybrids; These are the essential bases of a large part of the superfuel used in modern rocketry.
Boron does not burn at temperatures below 700 ° C.
The hydrides, such as the one of caborano, the pentaborane and the diborane, are destined to replace the carbon in their combinations with the hydrogen. Eldiborano is a toxic gas, very unstable in the presence of moisture or air. The technological processes required to obtain boron-based fuels are generally complicated. In practice, it is possible to stabilize the water and eliminate its toxicity, making it alkaline.
In the United States, the alkalinized pentaborane is known as Hi-Cal2 and Hef2, and that of caborano by Hi-Cal3 and Hef3. These fuels are used in the development of the X-15 rocket plane.
The fuels that use borane in their chemical composition have a great specific impulse, but they have a limitation. In fact, they can only be used in cases where air is available.
Thanks for reading this post, I hope it has been to your liking.
For more information, visit these pages:
https://ciencia.nasa.gov/science-at-nasa/2005/14oct_betterrocket
http://www.tecaeromex.com/esp/hibridos.html
@ jorge150785
nice
Thanks.
Thanks for this, I feel honored.
Interesting Post! Upvoted!