The common feature of
almost all compounds of the alkali metals is their high solubility in water.
All the compounds of alkali metals are ionic and so they have a giant ionic
lattice. As a result, they have high melting points and can be electrolysed
both in aqueous solution and as molten liquids. A few ionic compounds of
lithium with anions that have a large ionic radius have a small degree of
covalent character.
Starting with alkali metal oxides
The normal oxides of the alkali metals contain the O2- ion, and so are basic and react with water to give aqueous hydroxides – alkalis. Bases are proton acceptors, so the oxide ion accepts a proton from water to form the hydroxide ion:
O2-(aq) + H2O(l) →2OH- (aq)
Take sodium oxide, Na2O, as an example:
Na2O(s) + H2O(l) → 2NaOH(aq)
What about hydroxides?
Sodium hydroxide – commonly called caustic soda – Is a strong alkali. In water, it is fully dissociated into aqueous sodium ions and aqueous hydroxide ions and is referred to as a strong base. All the alkali metal hydroxides are strong bases and react with both weak and strong acids to give a salt and water:
OH- (aq) + H+ (aq) → H2O(l)
KOH(aq) + HNO3 (aq) → KNO3 (aq) + H2O(l)
Sodium hydroxide in qualitative analysis
In the laboratory, aqueous sodium hydroxide is used as a reagent to test for metal ions in solutions. Aqueous sodium hydroxide precipitates metal ions as their insoluble hydroxides. Very often, the hydroxides have characteristic colours that aid identification of the metal ion. For example, in the reactions of aqueous hydroxide ions with aqueous calcium ions, a white precipitate of calcium ion is formed, while with iron (III) formed a rust-red precipitate.

Image by janiceweirgermia from Pixabay
OXY-SALTS
The nitrates, sulphates and carbonates are much more thermally stable than those of other metals. The sulphates and carbonates are thermally stable a Bunsen-burner temperatures, with the exception of lithium carbonate. Even the nitrates decompose to form nitrites and oxygen rather than the oxide and oxygen. This contrasts with most other metal nitrates, which thermally decompose to give the oxide or the metal itself. Again, the exception is provided by a lithium compound, since lithium nitrate forms lithium oxide:
4LiNO3(s) → 2Li2O(s) + 4NO2(g) + O2(g)
Potassium nitrate thermally decomposes to form potassium nitrite:
2KNO3(s) → 2KNO2(s) + O2(g)
The alkali metals are the only group of metals that form stable, solid hydrogencarbonates. Sodium hydrogencarbonate is an important constituent in baking powder. It decomposes when heated to form sodium carbonate, carbon dioxide and water. The thermal stabilities of some of the carbonates and nitrates are described in more detail later in this post.
COMPOUNDS OF THE GROUP 2 METALS
With the exception of beryllium, most of the compounds of the Group 2 metals are ionic. Although it is a property of ionic compounds that they are soluble in water, some compounds are insoluble and there are distinct trends in solubility with similar compounds. For example, the solubilities of the carbonates and sulphates decrease, but the solubilities of the hydroxides increase as the atomic number of the Group 2 element increases.
What about their Oxides and Hydroxides?
The oxides and hydroxides of Group 2 elements are basic and neutralize acids. The oxide ion or the hydroxide ion reacts with aqueous protons to form water. The solubility of the hydroxides in water increases with increasing atomic number. Magnesium hydroxide is insoluble in water, whereas barium hydroxide is soluble. When water is added to the oxides, a comparable difference in solubility is observed, but this is because a chemical reaction takes place. For example, calcium oxide (quicklime) reacts with water to form aqueous calcium hydroxide or lime-water, which is sparingly soluble in water:
CaO(s) + H2O(l) → Ca(OH)2(aq)
When water is dropped slowly onto the oxide, slaked lime (solid calcium hydroxide) is formed:
CaO(s) + H2O(l) → Ca(OH)2(s)
Notice the importance of the state symbols in these two equations – without the symbols the equations would be identical, but they describe different processes.
Uses of the oxides and hydroxides
The basic properties of the oxides and hydroxides are used extensively to neutralize acids in a variety of applications. Slaked lime is used to neutralize acid soils and lakes. A suspension of magnesium hydroxide in water, called ‘milk of magnesia’, is used to cure indigestion.
Lime-water test for carbon dioxide
Aqueous calcium hydroxide (lime-water) is used to test for carbon dioxide. It gives a characteristic white precipitate often described as ‘milky’. This is a further example of the basic behaviour of the hydroxides, since carbon dioxide is an acidic gas and reacts to form insoluble calcium carbonate. When more carbon dioxide is bubbled into the white precipitate, it redissolves to form a colourless solution of aqueous calcium hydrogencarbonate.
OXY-SALTS
The oxy-salts (such as the nitrates and sulphates) of the Group 2 elements are white ionic substances. They show distinct trends in their solubility and thermal decomposition.
Sulphates
The solubility of the sulphates of the Group 2 elements decreases as the atomic number of the element increases. The aqueous barium chloride or barium nitrate test for sulphate ions rests on the insolubility of barium sulphate. A solution of the test chemical is mixed with aqueous barium ions and dilute nitric acid. The sulphate ion is present in the test solution if a white precipitate is formed and no sulphur dioxide is formed.
Carbonates
The carbonates of the Group 2 elements are all considered to be insoluble in water. Therefore, each can be prepared through precipitation by reacting a soluble carbonate, such as aqueous sodium carbonate, with a soluble salt of the element. The thermal stability of the carbonates increases with increasing atomic number of the Group 2 elements. Beryllium carbonate decomposes so easily that it cannot be isolated at room temperature. Magnesium carbonate decomposes at 540 °C and barium carbonate at about 1360 °C.
Calcium oxide (quicklime) is made in large quantities by the thermal decomposition of calcium carbonate (limestone). A temperature of between 900 and 1200 °C is needed, and it is important to remove the carbon dioxide produced, since the decomposition is reversible.
Nitrates
The nitrates of the elements of Group 2 are all white soluble solids that thermally decompose to produce the metal oxide, oxygen and nitrogen dioxide.
2M(NO3)2(s) → 2MO(s) + 4NO2(g) + O2(g)
where M = Mg, Ca, Sr, Ba and Ra. The thermal stability of the nitrates increases as the atomic number of the Group 2 elements increases. This trend is explained later on.
Talking of calcium carbonate, acid rain and FGD plants
Acid rain results from the presence in the air of sulphur dioxide and oxides of nitrogen. These gases react with the water and oxygen in the air to produce a mixture of acids including dilute sulphuric acid and dilute nitric acid. A major source of these gases is the power station that uses fossil fuels contaminated with sulphur.
Coal and orimulsion (a mixture of a tar and water imported from South America) are particularly rich in sulphur and cause most problems at power stations. Oxides of ritrogen are formed in the very high temperatures reached when the fossil fuels burn. They are sufficiently high to allow nitrogen and oxygen from the air to react directly to form nitrogen monoxide. When cool, the nitrogen monoxide reacts with more oxygen to give acidic nitrogen dioxide.
It has now become a high priority to remove these acidic gases from the waste or flue gases before they are emitted. A process called flue-gas desulfurization (FGD) is being introduced, in which cold waste gases are treated with powdered calcium carbonate. The acidic gases react with the calcium carbonate to give calcium nitrate, calcium nitrates (Ca(NO2)2), and mostly, calcium sulphite (CaSO3).
Almost 90 per cent of the sulphur dioxide and nitrogen dioxide produced by a power station can be removed by this process. The calcium sulphite produced is converted by reaction with oxygen into calcium sulphate, which is sold as gypsum and used in the building industry. The downside of the process is that large quantities of limestone have to be quarried to provide the calcium carbonate.

The atypical behaviour of beryllium compounds
Beryllium is not a typical Group 2 metal: in fact, it resembles aluminium in Group 3 more than magnesium in Group 2. The reason for this is the very small ionic radius of Be2+, which gives the ion a similar charge density to that of Al3+.
The beryllium ion, Be2+, is very polarising and distorts the electron cloud of any anion to which it is bonded. This results in either decomposition of the compound, as in beryllium carbonate (which decomposes even at room temperature) or the compound being covalent rather than ionic. Beryllium chloride is a covalent chloride with a layer lattice. This structure has each beryllium atom surrounded by four chlorine atoms, two bonded by dative bonds and two by covalent bonds. As a result the beryllium atom has a stable octet of electrons.
Beryllium hydroxide is also atypical in that it is amphoteric, whereas all the other hydroxides of Group 2 metals are basic, so it reacts with both acids and alkalis. Notice that in both the reactions a product is obtained in which beryllium has a coordination number of four, namely four groups around it that donate a pair of electrons to give a stable octet of electrons.
LATTICE ENTHALPY
To explain the thermal decomposition and solubility of an ionic substance, the forces that exist between positive and negative ions in its ionic lattice must be understood. Chemists estimate the strength of attraction between a positive ion and a negative ion in an ionic lattice from the enthalpy change called lattice enthalpy or lattice energy. Lattice enthalpy is the energy released into the surroundings when one mole of an ionic lattice is made from its constituent gaseous ions.
Therefore, the lattice energy is always negative. The more exothermic the lattice enthalpy, the greater the attraction between the positive and the negative ions. For sodium chloride, the lattice enthalpy corresponds to the following reaction.
FACTORS THAT AFFECT THE MAGNITUDE OF THE LATTICE ENTHALPY
The lattice enthalpy of each ionic compound is different and depends on the degree of attraction between the negative and the positive ions. This attraction depends on two factors: the ionic radius and the charge on the ion. The smaller the ionic radius, the greater the density of the positive or negative charge. This gives a more exothermic value for the lattice enthalpy. The greater the charge on the ion, the greater the attraction for an ion of the opposite charge. This also gives a more exothermic value for the lattice enthalpy.
Charge density
The ionic radius and the charge on the ion can brought together and treated as a single property of the ion called the charge density. The charge density is based on the assumption that the charge is spread over the outer surface of the ion, and defined as the charge per unit surface area. Although ions are not solid, we can still think of ions as having a boundary surface. The surface area is proportional to the square of the ionic radius, and so the charge density of an ion is given by:
Charge on ion = charge density / (ionic radius)2
BORN-HABER CYCLES
Experimentally, it is impossible to use a direct method to determine the lattice enthalpy of an ionic solid. So an indirect method that involves Hess’s law has to be used. Hess’s law states that if a change can be brought about by more than one route, then the enthalpy change for each route must be the same, provided that the starting and finishing conditions are the same for each route. Applying Hess’s law to the determination of lattice enthalpy, there is more than one route from the gaseous ions to the ionic lattice. The direct route and the indirect route shown in the figure below is known as a Born-Haber cycle. All the enthalpy changes in the indirect route can be measured experimentally. There are many ways to draw Born-Haber cycles.
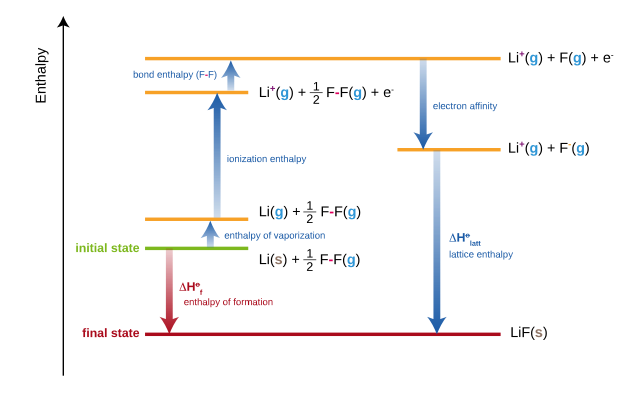
THERMAL DECOMPOSITION OF IONIC SALTS
The thermal decomposition of an ionic salt must necessarily involve the breakdown of one ionic lattice and the formation of another. For example, when magnesium carbonate is decomposed, it forms magnesium oxide and carbon dioxide. This requires that the lattice of magnesium carbonate is completely broken down followed by the formation of the lattice of magnesium oxide. It therefore follows that the enthalpy change that occurs during this decomposition must be related to the lattice energies of the two ionic substances involved.
DECOMPOSITION OF THE CARBONATES
The carbonates of Group 2 elements decompose as follows:
MCO3(s) → MO(s) + CO2(g)
where M = Be, Mg, Ca, Sr, Ba or Ra. As already stated, the thermal stability of the carbonates increases as the atomic numbers of the Group 2 elements increase. This trend is best explained in terms of the ability of the metal ion to polarise the carbonate ion and of the exothermicity of the lattice energy for the oxide formed.
Polarisation
Small, highly charged, positive ions can polarise negative ions. That is, they are able to distort the electron cloud of the negative ion. Negative ions that have a large ionic radius, such as the carbonate ion, are much more easily polarised than ions that have a small ionic radius, such as an oxide ion. Since the cations M2+ (where M is one of the Group 2 elements) all have the same charge, it is the ion with the smallest ionic radius that polarises the carbonate ion the most. Be2+ has the smallest ionic radius and so it causes most polarisation of the carbonate ion. Once the carbonate ion is highly polarised, it forms an oxide ion and carbon dioxide. This means that BeCO3 is the least thermally stable and RaCO3 the most thermally stable, since Ra2+ has the largest ionic radius.

MgCO3. Ondřej Mangl - Vlastní sbírka, Public Domain
Lattice enthalpy
An additional factor is the exothermicity of the lattice enthalpy of the oxide produced. This lattice enthalpy is more exothermic with a small cation than with a large cation. So, in the decomposition of magnesium carbonate, the formation of magnesium oxide with a highly exothermic lattice enthalpy can be considered as the driving force for the thermal decomposition.
Decomposition of the carbonates of Group 1
The carbonates of Group 1 metals are much more thermally stable than those of Group 2 metals, because the cation has only a single rather than a double positive charge. Lithium carbonate is the least thermally stable, since Li+ has a very small ionic radius, and hence a large charge density, and so can strongly polarise the carbonate ion:
Li2CO3(s) → Li2O(s) + CO2(g)
THERMAL DECOMPOSITION OF NITRATES OF GROUPS 1 AND 2
The thermal stabilities of the nitrates of Group 2 elements show the same trend as the carbonates: namely, the stability increases with increasing atomic (proton) number. The reason for this trend in thermal stability is again that the polarisation power of the M2+ decreases as the ionic radius increases;
2M(NO3)2(s) → 2MO(s) + 4NO2(g) + O2(g)
where M = Mg, Ca, Sr, Ba and Ra. Since the Group 1 nitrates have ions that normally are much less polarising, the decomposition products are the nitrites rather than the oxides:
2MNO3(s) → 2MNO2(s) + O2(g)
where M = Na, K, Rb and Cs.
In the next post, I will discuss on the solubility of ionic salts and other fascinating characteristics of the reactive metals.
REFERENCES
https://en.wikipedia.org/wiki/Sodium_hydroxide
https://www.chemicalsafetyfacts.org/sodium-hydroxide/
https://en.wikipedia.org/wiki/Alkali_metal_oxide
https://en.wikipedia.org/wiki/Alkaline_earth_metal
Alkaline earth metals-Chem.libretexts
https://www.britannica.com/science/oxide
https://www.britannica.com/science/mineral-chemical-compound/Oxides-and-hydroxides
https://www.education.com/science-fair/article/gas-sniffers/
https://chemstuff.co.uk/analytical-chemistry/tests-for-gases/
https://www.britannica.com/science/oxyacid
https://www.dictionary.com/browse/oxysalts
https://en.wikipedia.org/wiki/Acid_rain
https://en.wikipedia.org/wiki/Flue-gas_desulfurization
https://www.sciencedirect.com/science/article/pii/S0360128599000143
https://en.wikipedia.org/wiki/Beryllium
Hello,
Your post has been manually curated by a @stem.curate curator.
We are dedicated to supporting great content, like yours on the STEMGeeks tribe.
If you like what we are doing, please show your support as well by following our Steem Auto curation trail.
Please join us on discord.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.