 )
)The reason lies in the way the Universe, and later the Earth, first began. It is generally thought that the Universe started with tremendous fury in the Big Bang, just less than 14 thousand million years ago, when a super-hot dense 'soup' of sub-atomic particles exploded with unimaginable force. It was then that hydrogen atoms were first created. Some of these fused to give helium, and so a second element was born.
After about a thousand million years, stars began to form. Gaseous hydrogen with a little helium came together in local areas, contracting and becoming denser. The temperature rose to levels at which other light elements could form, and even more heat was produced. Stars continue to be born in this way from the dispersed material of the Universe.
The heavier elements appeared only in the relatively few very massive stars. Those with a much larger mass than the Sun became unstable after a few million years and exploded, propelling atoms out into the cosmos, to become the raw material of a second generation of stars with the heavier elements already formed.
It is thought that the Solar System was formed from such recycled materials about 4500 million years ago. The Sun itself is a gigantic fusion reactor, its heat and light helping to sustain life on Earth. The Earth contains some 90 light and heavy elements from the remains of earlier stars. In its intensely hot core, it retains some of the heat from when it was formed. More heat is produced by radioactive elements, which decay and release energy. In fact, as well as the heat of the core and from the Sun, this decay process is significant in maintaining the Earth's present-day heat balance.
A HISTORY OF IDEAS ABOUT THE ELEMENTS
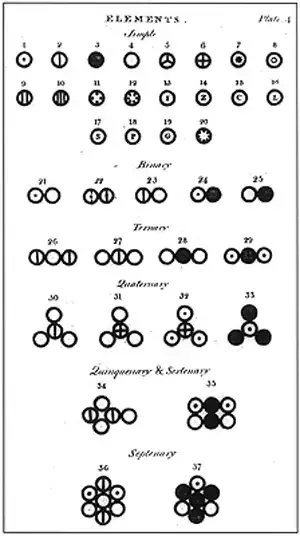
In 580 BC, the Ancient Greek philosopher Thales suggested that water was the fundamental 'element' from which all matter in the Universe was composed. Other Ancient Greek thinkers added earth, air and fire proposing that these four elements made up the world. 200 years later Aristotle added a fifth, 'aether', making up the heavens. This idea persisted for almost 2000 years until Robert Boyle published a book called The Sceptical Chymist in 1661.Boyle's book was a turning point in chemistry. It put forward the modern concept of an element as something that cannot be changed into anything simpler. The book also marked the beginning of chemistry as an experimental science, since Boyle was urging chemists to carry out practical investigations rather than merely observe, think and make deductions, as the Greeks had done.
 )
)His advice was heeded. In 1803, the English chemist, John Dalton, summed up 150 years of progress through experiment with his atomic theory. He used the Greek word 'atomos', which means 'cannot be cut', to describe the particles that make up elements. The idea that all matter was made up of tiny, indivisible particles had been proposed in 450 BC, again by an Ancient Greek, Democritus. But now, Dalton could support it with experimental results. These are the main points of his theory
• Atoms are indivisible and indestructible. • All atoms of the same element have identical mass and identical chemical properties. • When atoms react, they join together to form compound atoms' (now called molecules).Today, we still hold Dalton's view of an element as a substance composed of only one type of atom. So the element iron is made up of only iron atoms, which are different from copper atoms, which make up the element copper. We now know that an atom can be subdivided into smaller parts called sub-atomic particles, discovered after Dalton's time. But the atom is still the smallest particle with all the chemical properties of an element, and Dalton's atomic theory provided the foundation on which other scientists have built.
 )
) )
)BUILDING MODELS OF THE ATOM
A model is the scientist's attempt to explain observations. Dalton's model of the atom suggested that atoms could not be sub-divided into smaller particles. This model was accepted until the end of the century, when new discoveries and observations meant the model had to be changed.THE DISCOVERY OF THE ELECTRON
In 1897, J. J. Thomson was working at the Cavendish Laboratories in Cambridge. He was experimenting with a ray that was emitted from the cathode (the conducting material connected to the negative pole of a battery). This ray, called the cathode ray, had been discovered in the 1870s when an clectric current was passed through a gas at low pressure.Thomson found evidence that cathode rays were made up of particles which he called electrons. He calculated the mass of an electron to be 200 times less than the mass of a hydrogen atom and, as they were attracted to the positive anode, he knew they must be negatively charged. (In 1906, this work gained him a Nobel Prize.) Since these particles must be being produced from the atoms inside the apparatus, he realized he had discovered a sub-atomic particle. This meant that a new model of the atom was required to explain the observation.
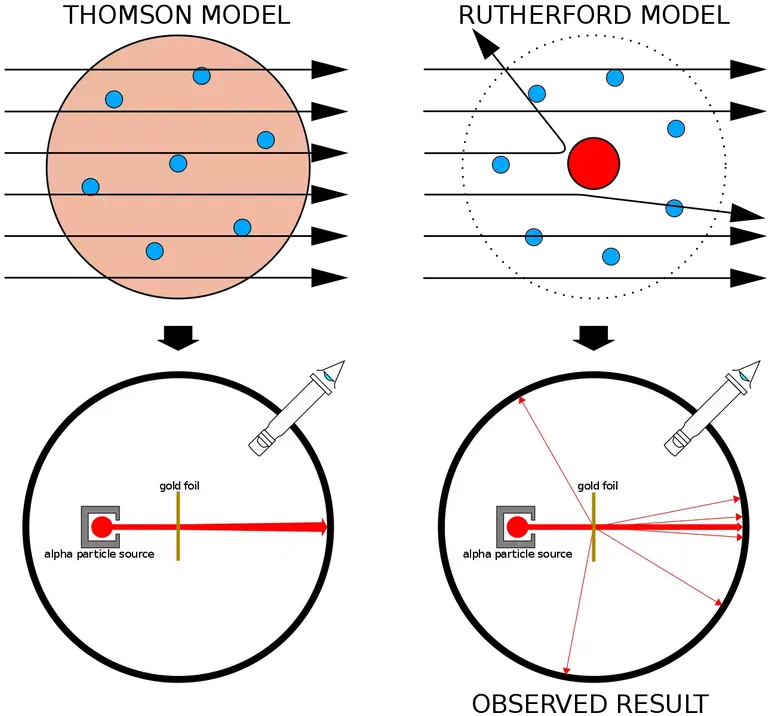
As no other sub-atomic particles had been discovered, Thomson's model of the atom was made up of negatively charged electrons. It was known that atoms had no net charge and were electrically neutral, so the electrons were thought by Thomson to be embedded in a sphere of positive charge, which had no mass. To account for the mass of atoms, Thomson assumed that they must all have thousands of electrons. His model was known as the 'plum pudding' model because the electrons were pictured like raisins in a pudding.
 )
) )
)RUTHERFORD'S MODEL OF THE ATOM
Ernest Rutherford, a New Zealander working under Thomson at Cambridge began investigating a newly discovered phenomenon, called radioactivity. Over several years of work, he found that the radioactivity, which a French scientist called Henri Becquerel had first observed, was composed of three main types of radiation. Rutherford named the first two alpha (α) and beta (β) radiation, when the third was eventually discovered, it was called gamma.Rutherford showed that alpha radiation was composed of particles that were four times heavier than hydrogen. In fact, they are the same as positively charged helium atoms. (We refer to them as helium nuclei.) The beta particles were soon found to be very high-energy electrons, which of course are negatively charged. Gamma rays were identified later as electromagnetic rays similar to X-rays, but of much higher energy. Being electromagnetic rays, gamma rays were found to have no particle properties, and therefore no mass.
RUTHERFORD'S SCATTERING EXPERIMENT
Rutherford moved to Manchester University to set up his own research group which included Hans Geiger and Ernest Marsden. In their investigations into the structure of the atom, they fired alpha particles at gold foil. From Thomson's model of the atom, it was expected that a few alpha particles would be deflected slightly from a straight route when they passed through foils. Even the thinnest foils were about 2000 atoms thick, and some of the alpha particles were sure to collide with electrons. Marsden was asked to see if any alpha particles were scattered through large angles. Rutherford said, 'I may tell you that I did not believe they would be, since we knew that the alpha particle was a very fast, massive particle, with a great deal of energy.But then, a few days later Geiger announced that some alpha particles had been recorded as bouncing back from the gold foil. Rutherford recalled, it was quite the most incredible event that has happened to me in my life. It was almost as incredible as if you fired a fifteen inch of artillery shell at a piece of tissue paper, and it came back and hit you.' The results of the scattering experiment are a good example of how science works. To check the reliability of the results, the experiment was repeated many times.
It was now clear to Rutherford that Thomson's model of the atom may not be correct. If it were, then the alpha particles should have shot through the foil with hardly any deflection. But one in 8000 had apparently been halted in its tracks and deflected back. Most particles passed through without any deflection, and Rutherford concluded that they were travelling through empty space. The positively charged alpha particles deflected through larger angles must have encountered (and been repelled by) another positive charge of considerable mass, which he called the nucleus.
 )
) )
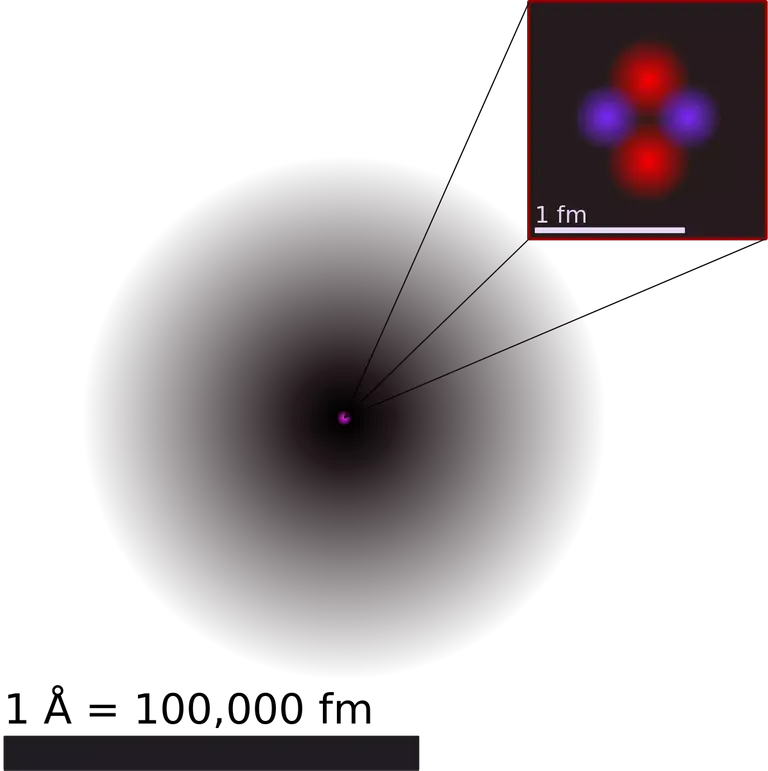
)From the angles of deflection and other data, Rutherford calculated that the radius of the gold nucleus was 10-14 metre. (We now know that the radius is closer to 10-15 metre.) He calculated, too, that the radius of the atom was approximately 10-10 metre. This means that the nucleus was 100 000 times smaller in diameter than the atom - an astonishing difference. If a full stop on page represented the nucleus, then the outer limit of the atom would be about metres away. No wonder that only a very few alpha particles came back.
Rutherford proposes his model
Rutherford now proposed his model of the atom which is a very small, central nucleus containing all the positive charge of the atom and almost all of the mass, surrounded by empty space in which electrons orbited the nucleus, rather like planets round the Sun.This model of the atom raised some difficult questions. In particular, since opposite charges attract, why did the electrons not fall into the nucleus? The Danish scientist, Niels Bohr, came up with the answer to this question which I'll be discussing in full in my next post. The idea of the minute, central, positive nucleus is fundamental to all models of the atom accepted today
PROTONS AND NEUTRONS
The proton was the next sub-atomic particle to be discovered, and again the alpha particle was the tool used in research. When alpha particles were fired through hydrogen gas, positive particles emerged which were about the same mass as the hydrogen atom. In 1919, Rutherford said that these same particles could be knocked out of other atoms, suggesting that they must be present in the other atoms. He gave the name protons to these positive particles. The model of a nucleus made up of protons was now taking shape.A year later, Rutherford suggested that protons were not the only type of particle in the nucleus, and that there must be particles of equal mass but neutral charge in the nucleus as well. In suggesting this, Rutherford was almost a lone voice in the scientific community. Then, in 1932, James Chadwick, another member of his research team, was able to show that this neutral particle did exist.
 )
) )
)Chadwick fired alpha particles at beryllium atoms. Instruments that detected charged particles were unable to detect any radiation. Yet when paraffin wax was put between the beryllium and the detector, a shower of protons was detected. Rutherford guessed that the protons were coming from the paraffin because some sort of radiation was hitting it. He said this radiation was like the effect of an invisible man who cannot be seen directly, but who is known to be there because he collides with other people in a crowd.
The invisible particle in the radiation of Chadwick's experiment - with no charge and with its mass equal to that of the proton - was named the neutron. In this way. protons and neutrons became known to scientists.
Very nice article. What are you working on in your offline life? Are you a scientist? I was away from Steem during the last month and I am happy to have read your post as the first post after my return :)
This being said, I have a few comments on the first part of the post.
Strictly speaking, hydrogen formation did not happen at the time of the big bang, but during the first seconds of the life of the universe (which is thus after the big bang).
Stars began for form only 1.6 million years after the big bang thanks to gravity.
I do research when I'm not online reading science journals. Yes, I'm a scientist; a chemist to be specific.
Thanks, for coming by and for your good "few" comments. I appreciate. And I'm glad you found my post interesting to read. Be on the look-out for more.
Two is a few, isn't it ? :)
I didn't say it but I always like to read about science history. This always makes me smile about how we evolve to what we know today.
Very interesting overview. Thank you!
Thanks for coming by.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Please consider setting @steemstem as a beneficiary to your post to get a stronger support.
Thanks for having used the steemstem.io app. You got a stronger support!
I observed the use of Alpha Particles especially in Rutherford's experiments, discovery of Protons and in Chadwick's experiment also.
What are these Alpha Particles, and why were they preferred?Well done @empressteemah ... This is my first read in a long time. Interesting topic it is.
Baba oh.... It's been a while oh. I've missed your great posts on microbiology. When are you dropping one? I'll be on the look-out.
My boss, I salute you!... Hehe, "great posts!" flattering me bah? Lol, I should drop one soon. Thanks brother, I have missed this great platform.
Thanks, @herbayomi. I just went through one of your post now. Nice narrative style.
Alpha particles can simply be called high speed particles made of two protons and two neutrons binded together by a very strong nuclear force. Its nucleus is the same as that of the atom of helium. A very fast traveling nucleus that has no electrons. It's categorized under ionizing radiation.
Alpha particles are preferred in most of these experiments because they are stable and travel very fast with a great deal of energy. It is said in many textbooks that alpha decay involves emitting alpha particles, which are very stable.
Thanks, for coming by. I'll be looking forward to more of your comments.
A detailed response and additional information, thanks for this Empress.
So, I have learnt that the nucleus of an alpha particle is identical to that of helium, and that because of its relative stability, speed in motion and energy capacity it was preferred for those experiments highlighted in this article.
Thanks for the compliment. Looking forward to more of your interesting articles.
Yes, u're welcome @herbayomi. Hope to see you on stemng discord server.
A very great article, @empressteemah. Keep it up. I'll be looking forward to many more posts from you.
Thanks.
Thanks, @emperorhassy.