Note: the images can be a little disturbing for some people.
For years, multiple medicaments have been created with the aim of treating and curing diseases that have put human life at risk. The medical area is very wide and there are infinities of pathologies that if they are not well treated could put in risk the life of the patient. The next topic that I will show, is about the use of oral anticoagulants especially about the use of vitamin K antagonists such as Warfarin and acenocumarol, its mechanism of action, pharmacodynamics, pharmacokinetics, indications, contraindications and adverse effects.
Both endovenous and subcutaneous oral anticoagulant drugs, according to their presentation, have been very useful in the medical field. Its use has been for more than 100 years, each one has had its own history, with its qualities, effects and indications according to the pathology to be treated with numerous studies and tests on individuals over time.
The heparins of high weight and low molecular weight, have their controversial history according to its review its creation dates from 1911 at the hands of the scientist Doyon while other literatures describe it since 1916 by Jay Mc Lean where the latter discovered that cephaline and heparanphosphatide as they aged lost their procoagulant property and were transformed into active anticoagulants thus obtained to heparin, This being the beginning of many more studies to come to give way to the era of anticoagulants being the last to be created in 2008 called the new oral anticoagulants.
Under normal conditions the blood must maintain its fluidity within the vascular bed and still coagulate quickly and effectively when the surface of the endothelium is exposed to vascular injury, thus maintaining a balance between coagulation and fibrinolysis which prevents the formation of thrombus.
The purpose of this type of drug is to provide blood flow in the circulation, has various indications both prophylaxis and therapeutic thromboembolic pathologies that may be products of major surgeries, secondary hypercoagulation states to neoplastic diseases, heart disease among others.

When triggering injury or damage to the surface of an endothelium with vascular compromise, the body employs multiple mechanisms to maintain the integrity of the circulation.
As there is a lesion capable of causing blood extravasation, a series of substances are activated that will give way to primary and secondary haemostasis for educational purposes as these processes occur almost simultaneously which will be described below.

It is the first step that basically consists of local recruitment and platelet activation that will form the platelet haemostatic stopper, through three phases: adhesion, activation and secretion by final aggregation.
In this first phase of platelet adhesion in the injured endothelium is mainly given with collagen by von Willebrand factor (FvW) and GPIb/IXa. This union allows the beginning of the next phase of activation and secretion mediated by own active substances (adenosine triphosphate, platelet factor 4, calcium, serotonin, growth factor derived from platelets, thromboxane A2, factor V-VII and fibrinogen) that will favor the aggregation and therefore platelet clot formation and tissue repair.

The very complex one which involves the factors of the coagulation, proteins and diverse substance that will give step to what nowadays we call cascade of the coagulation, with its last update approximately 8 years ago, where what was believed to be an intrinsic and extrinsic way is already only a common way for this cascade.
This is divided into 3 phases called: Initiation, Amplification and Propagation.
Initiation phase, mediated by tissue factor or previously called thromboplastin or factor III located in the cell membrane for this to be activated there must be endothelial damage that allows the plasma to come into contact with this factor, then factor VII is free looking for a lesion to activate and start this cascade. The factor VII forms a complex of union with the tissue factor giving origin FT/VIIa, which activates the factor X and IX. Finally, activated factor X binds with activated factor V and, in combination, facilitates the synthesis of essential thrombin in the propagation and amplification phase.
Amplification phase, basically the small amounts of thrombin that are produced in the first phase, are enough to generate platelet activation giving rise to a prothrombotic period. During this phase, platelets are capable of producing alpha granules on the endothelial surface, where the activated partial factor V generates more thrombin, which is why this amplification phase is called. On the other hand, the Von Willebrand factor (FvW) will be activated almost simultaneously, with the objective of activating factor VIII.
Propagation phase, once activated the factors already mentioned in the first phases (XI, X, V, VII, VIII) where the factor IX activated together with the factor VIII already activated adhere to the platelet membrane and form the "ten-ase" complex that activates more factor X and calcium. This activated factor X that is already in greater quantity will allow the assembly of the complex of prothrombinase, which transforms the prothrombin to thrombin, where the latter generates the release of fibrin and formation of the clot.
When the coagulation cascade is carried out, another process called plasmin-mediated fibrinolysis must be carried out, whose objective at this stage is to remove clots during wound healing and also to remove intravascular clots that may manifest as thrombosis.
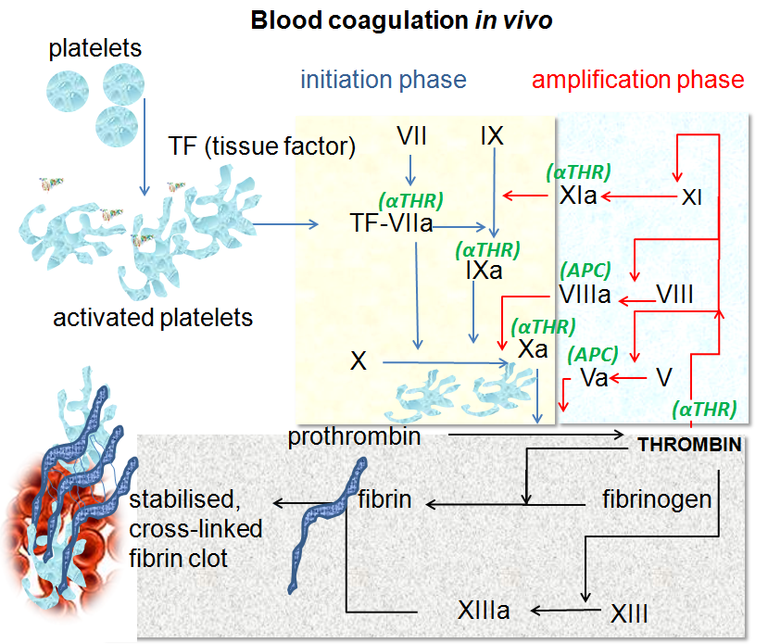
Blood coagulation pathways in vivo. Dr Graham Beards. CC BY-SA 3.0. Wikipedia


I can say that heparin is a glycosaminoglycan located in the secretory granules of barley cells, which is synthesized from UDP-glucose. Heparin is naturally extracted from the intestinal mucous membrane of pigs as this area is abundant in barley cells.
Heparin derivates are those that are currently useful in the medical field, and these include high molecular weight heparins, low molecular weight and fondaparinux.
Its mechanism of action is obtained when it binds to the antithrombin by means of a sequence of Penta saccharide which contains a 3-O-sulphated glucosamine residue. Therefore the low and high molecular weight heparins together with fondaparinux act catalytically.
The desired pharmacological effect of these drugs is to interfere with platelet aggregation and prolong clotting time. However, although these three drugs are derived from heparin, they retain different properties and characteristics, i.e. high molecular weight heparins such as sodium heparin, are larger in size and therefore have a greater anticoagulant effect by encompassing and inhibiting tissue factor, thrombin and factor Xa. Whereas the heparins of low molecular weight for being a shorter chain tends to inhibit with greater affinity to the factor Xa in relation to the thrombin with less effect.
These medicaments do not cross the placental barrier, which is why their use during pregnancy is approved. It is administered parenterally in the case of high molecular weight heparin and subcutaneous for low molecular weight and fondaparinux.
Acenocumarol and Warfarin belong to this group of anticoagulants whose exclusive administration is orally. Its mechanism consists in inhibiting the conversion of vitamin K oxidized to its reduced form, where the latter is essential in the hepatic synthesis of the so-called k-dependent proteins and these are: prothrombin, VII, IX, X and protein C and S.
In addition these antagonist drugs have the ability to decrease plasma levels of prothrombin works, which enhances its anticoagulant effect.
Because it is liposoluble, it absorbs easily and quickly into the digestive tract, which is why it is administered orally, and which in turn allows it to easily cross the placental barrier caused by the teratogenic effect in the first trimester, contraindicating its use in these situations.
The half-life of acenocumarol is approximately 9 hours so it is indicated from 12 to every 8 hours, compared to Warfarin with a half-life of 36 hours and is administered day order.

Heparins and their derivates in conjunction with vitamin K antagonists have similar therapeutic uses such as:
Atrial fibrillation, pulmonary embolism, acute myocardial infarction, deep venous thrombosis, coronary angioplasty, systemic embolization in individuals with valvular prostheses, major hip and knee prostheses, especially prophylactic.

Heparins and their derivates are more frequently associated with hemorrhages that can be simple as epistaxis and severe as digestive hemorrhages.
Thrombocytopenia induced by heparin, alteration of hepatic functionalism and osteoporosis. The control of coagulation with these drugs is performed from the TPT and in cases of heparin poisoning secondary to serious dose Protamine.
While Warfarin and Acenocumarol, hemorrhagic manifestations ranging from simple to complex are also observed with frequencies. Congenital alterations during consumption in pregnancy.
Warfarin-induced skin necrosis is a rare complication characterized by the appearance of skin lesions three to 10 days after starting treatment.
Another type of lesion that can be observed at skin level are painful blue lesions located on the plantar surfaces and lateral faces of the toes, which disappear with pressure and elevation of the legs. Cases of alopecia, urticaria, dermatitis, anorexia, nausea and vomiting have been reported.
The control of coagulation is made by means of TP and its antidote in cases of intoxication is the vitamin k.
To determine that a patient is in anticoagulation range must have an INR of 2 to 3.

Factor Xa inhibitors
In this group we have Rivaroxaban, Apixaban, Edoxaban and Betrixaban, at the level of the coagulation cascade act by inhibiting the activated factor X associated with thrombin. It is administered orally once a day and does not require continuous monitoring of coagulation time (Tp-TpT and INR).
Their therapeutic use is similar to heparins, as they are useful in the treatment of deep vein thrombosis, major surgical interventions such as hip replacement, as well as prophylactic drugs for DVT in patients with cancer, or bedding, prior to orthopedic interventions among others.
Thrombin Inhibitors
The only drug available for now is Dabigatran, its mechanism is given by behaving as a competitive antagonist reversibly blocks the active site of thrombin. Its plasma half-life is 12 hours, for this reason it must be administered twice a day. Like factor Xa inhibitor drugs, they do not require control of clotting times. And its use is contraindicated in pregnancy and lactation as it crosses the placental barrier and is excreted with breastfeeding.

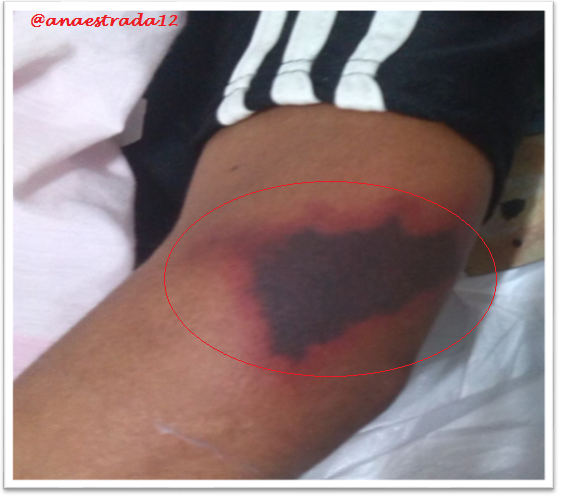
This is a 32-year-old male patient with no known pathological history who has deep vein thrombosis of the left lower limb. 2 months prior to which he receives oral anticoagulation with antagonist drugs of vitamin K Warfarin type, which suspends for 6 days, showing extensive erythematous lesions, initially reddish and finally crusted lesions of purplish-black, painful on palpation, located in both elbows and left knee, accompanied by sero-hematic secretion. In relation to the clinical findings together with the history of Warfarin ingestion, with clinical manifestations a few days after the suspension of the drug, culture and lesion biopsy are taken, the result of which affirms the presumptive diagnosis of Warfarin-Induced Cutaneous Necrosis.
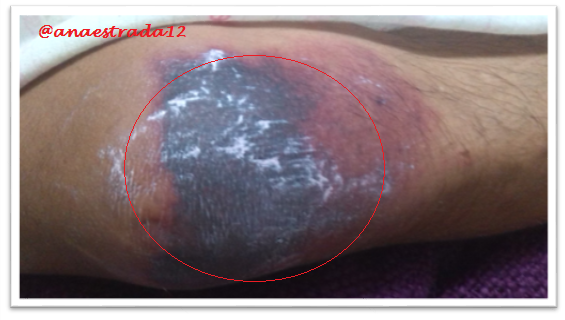
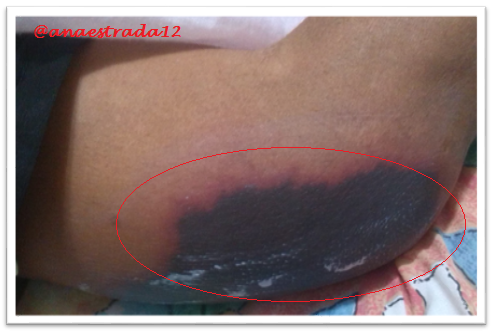

Warfarin-induced necrosis is an infrequent complication but described in the literature since 1943, which was initially known as "disseminated thrombophlebitis migrans" and it was not until 1954 that the name of warfarin-induced necrosis is given after 13 cases reported for that time.
Cutaneous necrosis occurs at any age, as long as you are treated with Warfarin. The affected areas in order of frequency are generally sites with thick subcutaneous fat such as breasts, buttocks, thighs, arms, hands, fingers, legs, feet, face, nose and abdomen. In men the most affected area is the penis.
In approximately 35% of cases, the lesions can be multiple and symmetrical, such necrotic lesions usually appear suddenly between the third and sixth day from the beginning of the administration of oral anticoagulation.
The initial manifestation is of paresthesia, sensation of pressure and pain, then appears a hardened area, erythematous and localized, with a rapid evolution to petechiae and ecchymosis with formation of a hemorrhagic blister that finally culminates with necrosis of the affected area. Very similar to the clinic described in the exposed case report.
Physiopathologically, the hypothesis used for this complication is that when the anticoagulation is suspended, the levels must have decreased temporarily or a procoagulant state (sepsis, heart failure, hepatopathy, for example), and once the anticoagulation has restarted, the necrosis develops. This is possible since protein C, which is synthesized by the liver, has a short half-life and its concentration in blood would be reduced in those pathologies that affect the liver, it could even occur by interaction with other drugs that lower serum levels or increase the metabolism of Warfarin, such as the use of salicylates during four days prior to the event, possibly by displacement of Warfarin bound to albumin, originating in a transitory procoagulant state.
However, a transitory imbalance between anticoagulant-procoagulant systems appears to occur, associated with a rapid decline in protein C levels during initial Warfarin therapy. Likewise, it is not ruled out that a hereditary factor such as the hereditary deficiency of protein C may be involved. Resistance to activated protein C is found in 5% of the general population, but in the vast majority of cases, Warfarin-induced necrosis is not associated with Warfarin deficiency.
Once the diagnosis is suspected and confirmed, Warfarin should be discontinued, treatment with unfractionated heparin or low molecular weight heparin should be started, to avoid the formation of other thrombus, the use of vitamin K and fresh frozen plasma is advised for a rapid replacement of the levels of protein C and S.
Cover Images


steemSTEM is a project of the chain of blocks that supports the scientific content in different areas of science. If you want to know more about this wonderful project you can join the server in discord
This article will be published at https://www.steemstem.io/

link
I hope you enjoyed my content.

If this is not treated in time could it cause the loss of the leg? or am I exaggerating?
Hello Carlos, this type of injury is directed at the skin as such, and its more superficial layers, causing necrosis of the entire limb even its bony portion has not been described. Thank you for reading the article.
Thank you for clearing up my doubt DRA.
It’s very interesting and great explaned !
Posted using Partiko iOS
Thank you very much, for me it is a pleasure to share medical content
Are you a Doctor or do you work in the medical world ?
I'm a doctor..
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
Thank you very much