Ceramics and Refractory products
Ceramics are products widely used across the globe that do not get as much recognition that they should. The reason I say this is because these products are the few materials in the world that can be manufactured and used at extremely high temperatures, thus providing uses in UHT processes as well as around an open fire. The word ceramics originates from the Greek word keramikos which translates to “burnt stuff”. Desirable ceramic products are composed of inorganic, non-metallic material and produced through a high temperature heat treatment process known as firing. These products are composed of a simple compound of silicon that is chemically attached to a variety of non-metallic element on the periodic table such as CON (carbon, oxygen and nitrogen). Although it is most ideal for only non-metallic structure to be present, there are a few cases when metallic compounds are present in the products chemical construction.
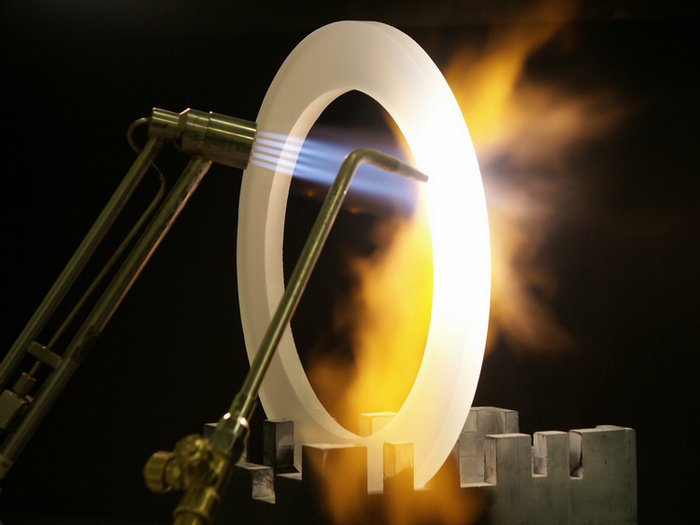
Heat resistant property of ceramics<>
Ceramic products, although mainly renowned for their [high temperature resistance (https://en.wikipedia.org/wiki/Thermal_resistance) have a lot of other, equivaluable properties. This includes toughness, having a strong and brittle structural integrity, having a high melting point, high compressive strengths, a low conductivity in terms of electrical and thermal properties, and a good stability in chemical and once again thermal properties.

Inner lining of a furnace<>
A few simple chemical compounds of ceramics are MgO (magnesia), SiO (silica), Al2O3 (corundum), Si3N4 (silicon nitride), SiC (silicon carbide), cristobalite – bricks used as furnace linings due to their extremely high temperature resistance and even quartz – used as an abrasive such as commercial sand paper. Examples of a few complicated ceramic compounds are in a variety of clay types, including kaolinite, dolomite, mullite, feldpar, calcite and many more.
From ceramics, comes a branch of new products that are said to have higher heat resistant products. These are referred to as refractory product. The word refractory comes from the word “Refractoriness” which is the property of a material to not deform during service while extremely high temperatures are inflicted upon it. The criteria of refractory are based upon a materials melting point or the rate of softening of the material. A few examples of refractory products include FeO (Wüstite) with a melting point of 1400° C, CaO (Lime) with a melting point of 2570° C, Al2O3 (Corundum) with a melting point of 2020° C, ZrO2 (Zirconia) with a meting point of 2700° C and even Cr2O3 (Chrome Oxide) with a melting point of 2280° C.
Refractory products are not the only branch of ceramics there are. There are:
• Glasses
• Clay products
• Refractories
• Abrasives
• Cements
These branches can be branched further and will be identified.
Glasses
Glasses can be split into 2 sections: general glasses and glass ceramics. Both these sections of glasses are non-crystalline silicates that combine with other compounds and contain oxides such as CaO, K2O and Na2O.
An example to illustrate the complex composition of glass is as follows:
If we use fiberglass as an example, we may notice that its chemical makeup consists of (in percentage mass (%) ), 55% SiO2, 16% CaO, 15% Al2O3, 10% B2O3 and 4% MgO.
Most glass structures contain these intricate chemical nature as they have highly intensive and specific work to do. Vycor needs to be very resistant to heat as it is used in laboratories, optical flint needs to have a high density and a high refractive index, pyroceram needs to be strong, easily fabricated and thermal resistant. For these requirements to be met, we need to make sure the chemical structure of glass is solid.
Other uses of glass in everyday use includes containers, lenses for eyewear, windows and even inner lining of certain reactors and home appliances.
The main characteristic to look out for in glass and glass ceramics is the following:
It must have a low coefficient of thermal expansion, a high thermal conductivity and a relatively high level of mechanical strength.
Clay products
Clay is one of the most widely use ceramics in the world. The reason for this is due to the leading fact that it is a cheap material in construction and found in great abundance. This material does not require reforming in any way and is most often used as it is mined. It is also an easily malleable material but it does contain moisture which may degrade its strength. To overcome this obstacle, a process known as firing or fired was introduced. This removes most of the moisture from within the piece and improves its mechanical strength also.
Clay can also be split into 2 separate groups which are structural clay products and whitewares. Structural clay products are products used in construction where structural reliability and integrity are most important. They are used in the production of sewage pipes, floor and roof tiles, and even building bricks. Whitewares on the other hand are more commercial products of clay. They get their name due to their appearance after firing. The final products include pottery, tableware, plumbing fixtures and even porcelain tiles.
Refractories
Refractory products can be split into 4 classes of refractories. These include:
Fireclay refractories, Acid or Silica refractories, Basic refractories and Special refractories.
Fireclay refractories are products that have a chemical composition that mainly consist of alumina, high purity (free of pollutants) fireclay and silica, with alumina being the main ingredient as it constitutes between 25 – 45 weight percentage of the overall product (%). The main uses of this type of refractory is to thermally insulate structures from excessive temperature to prevent any form of deformation and thus confining a hot enclosed atmosphere. It is mainly used as bricks during furnace construction and we can understand why due to its characteristically work description.
Acid or Silica refractories are stronger than that of the aforementioned due to this branch having the ability to not only withstand these exorbitant degrees of heat, but also being able to do this under a load-bearing capacity. Its primarily important ingredient is Silica and is used in the arched roofs of furnaces such as steel and glass making, where temperatures reach an excess of 1650°C. Since this refractory is resistant to slag that contains silica, also referred to as acid slag (hence the name of this refractory), it is also used in containment vessels to house this acid slag.
Basic refractories were given its name because it has the ability of a general refractory product and does not contain a special ability like the others. It is used in the walls of an open-hearth furnace as these furnaces do not reach extremely high temperatures above 900°C. It consists of many iron compounds and includes carbon and chromium. It must not be mixed with silica however, as silica will have an adverse effect on the temperature resistant property of this refractory. This refractory is also used for containment of slag with a high concentration of CaO and MgO.
The last type of refractory is referred to as Special refractories, and this is because these products have certain properties that allow them to be used for specialized refractory uses and applications. This is the most expensive branch of refractories as they contain a large span of compounds and elements in its constitution which include magnesia, beryllia, zirconia, and carbide molecules along with graphite and many more compounds. Silicon carbide, which is one of the compounds, is used because it has a high resistance to electrical heating as well as heat resistance. These properties makes this refractory as well developed material and most sort out for when it comes to internal furnace design and attributes.
Abrasives
Abrasive ceramics are the only few products that occur naturally but also be synthesized. Abrasives are used to wear of other material or even grind off other material that are softer to that in comparison. To carry out these tasks, these ceramics need be hard as well as have a high resistance to wearing off itself thus prolonging its use, it must be tough so that during application the abrasive to be used does not crack or fracture, and lastly, they must have a certain degree of refractoriness as high temperatures may be produced due to frictional contact between the 2 or more materials. The most common and strongest natural abrasive known to man are diamonds. They are used as abrasives around the world and can only be cut using another diamond, thus reinforcing is strength. The downfall however is the fact that it is very expensive to buy and use and often not feasible to many. To solve this problem, scientists and chemist created their own cheaper abrasives that have comparable strengths to that of our ideal, the diamond. These include tungsten carbide, corundum, silica sand and more.
Cements
Cement is widely used around the world as it is most reliable and the building blocks in many industries such as architecture and construction. Cement creates a paste when amalgamated with water and sets and hardens to create a steady, strong base of material bound together as one as it used as a bonding structure to chemically bind materials into a single cohesive structure. It is extremely malleable thus providing an advantage as it can form infinite shapes and sizes for every nook and cranny.
There are many types of cements used worldwide, but the most widely used type is known as Portland Cement. It is produced by mixing grinded clay and minerals composing of lime, in the correct proportions and escalating the temperature of this mixture to roughly 1400°C through calcination. The clinker will then be smashed and ground into a fine powder and with the addition of gypsum, Portland cement is made.
All branches of ceramics are important but the most important type due to its everyday use as well as versatility is glass. Due to this fact, it was decided by chemists to implement certain tactics to make sure that glass would have added toughness and extra features to prolong its span. There are 3 main strategies, annealing, thermal tempering and chemical treating.
Annealing- ceramic materials are heated to a high temperature and immediately cooled down again to below room temperature. This is done to introduce thermal stresses, which is a result due to a contrast between that of cooling rate and thermal expansion and contraction. Thermal stresses are important in glassware as it may strengthen a material but may also cause it to fracture or break so it must be done carefully. To avoid large amounts of thermal stresses, the glass is cooled at an extremely slow rate. If larger amounts of stresses are added however, we reduce them by an Annealing heat treatment. The glassware is heated to the annealing point and then cooled down slower than usual, thus, reducing the stresses and strengthening the glassware itself while cooling.
Glass tempering- the strength of glassware can be improved by creating compressive residual stresses at the surface of the glass. The process occurs as follows:
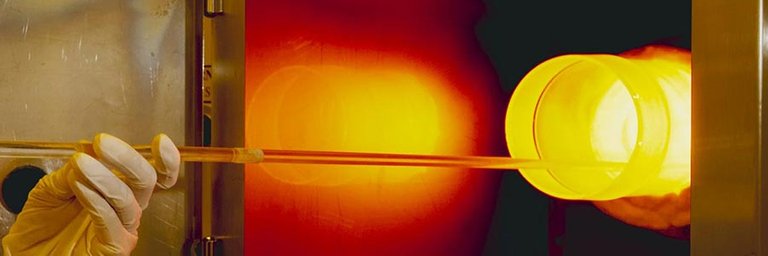
Heat is added to the glass product provided but there is a range for the amount of heat to be added, it must be above the glass transition region but below the softening point of the glass, as each glass product has its individual properties. From this elevated temperature, it is cooled to room temperature by the use of either a gush of air or through quenching. Due to glass having a certain thickness, residual stresses will occur, which results due to a difference in cooling rates of the surface and the interior as the surface will cool mush faster. Since the exterior cools faster than the interior, the outside becomes rigid while the inside becomes plastic. The interior will attempt to contract to a larger extent than the rigid exterior will allow and in doing so, the interior will tend to prevent inward radial stresses caused by the surface. After this glass product reaches its desired temperature, it has both compressive and tensile stresses. For a fracture to now occur upon the product, a force that is greater than both the stresses must be applied upon the product. Thus, the product has been strengthened. Tempered glasses will be used when application of high strength is required. Optimization of strength though tempering depends on the temperature achieved as well as the rates of cooling, the coefficient of expansion and the size and number of flaws already present in the given glassware.
The last type of treating is called chemical treating. The glass is heated at optimized at an increased temperature to increase its strength and sustainability as well as provide other beneficial additives. Its applications include gorilla glass for cellular devices, self-cleaning glass (containing a coating of TiO2 to burn of dirt molecules when activated by the sun) and even self-tinting glass (containing structures that use UV light as an indicator to change the glasses structure to a “tint”). This is a reversible process and loss of UV light counters the original statement.
The End
Images are linked to their sources in their description
References:
[1]http://glassed.vitroglazings.com/glasstopics/heated_glass.aspx
[2]https://en.wikipedia.org/wiki/Ceramic
[3] www.explainthatstuff.com/ceramics.html
[4]https://www.scientificamerican.com/article/how-is-tempered-glass-mad/
[5]https://www.linkedin.com/pulse/types-refractory-materials-applications-le-sylvia
[6]iti.northwestern.edu/cement/monograph/Monograph3_8.html









Why is everything centered and italicized?
Hello sir. I was actually trying to create my own look for my posts to help with individuality but I can understand that this wrong and not an accepted format. I am sorry for this inconvenience and it will not happen again.