Fuel Cell: Basic Operation
A fuel has chemical energy. In history, we had been using fuel to get energy by burning it, but in a fuel cell we convert chemical energy into electrical energy by electrochemical reaction. Batteries also work in same energy conversion principle. The difference is that batteries contain reactants in itself but in a fuel cell, we have to supply the fuel continuously. Fuel cells are now used in automobile, aviation, solar, submarine, smartphones etc. Some advantages of fuel cells are:
i. Fuel cells release very low or no pollutants. The products are electricity, heat, and water.
ii. Fuel cells have higher Efficiency
iii. There is fuel flexibility in a fuel cell.
iv. The operation of a fuel cell is quite.
v. Fuel cells are scalable i.e. they can be stacked.
Construction
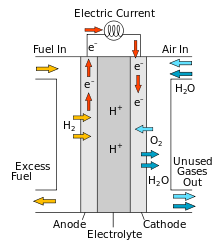
(credit: https://en.wikipedia.org/wiki/Solid_oxide_fuel_cell)
Fuel cells consist of anode, cathode, electrolyte, load, connecting wires and membrane. An anode is an electrode where oxidation takes place and the cathode is an electrode where reduction takes place. An electrolyte is a liquid solution which allows movement of ions. The membrane used in a fuel cell is proton exchange membrane which allows the passage of proton only. Connecting wires are made up of copper for the flow of current and load is where electricity is needed and consumed.
Operation
At first, hydrogen fuel enters in an anode and oxygen fuel enters in the cathode. Loss of electron is oxidation and gain of the electron is a reduction.
Source
In anode:
Proton exchange membrane allows passage of proton i.e H+ only. The electron flows from an external circuit.
In cathode:
The by-product of fuel cell is water. Due to the electrochemical reaction, about 0.7 V potential difference is created and electric current flows continuously through the wire. Fuel cells are stacked or compiled for large voltage generation which is called fuel cell system.