Did you know? There is an insect that walks on water; a steel pin that floats on water Not only that, the raindrops are spherical in shape, a drop of mercury is also spherical in shape (well, almost) and can be rolled just like a steel ball(looks the same too!). All these observations can be understood from the concept of ‘surface tension’. This idea may be new to you so, I will try my best to explain this concept from the ground up.

(www.pixabay.com)
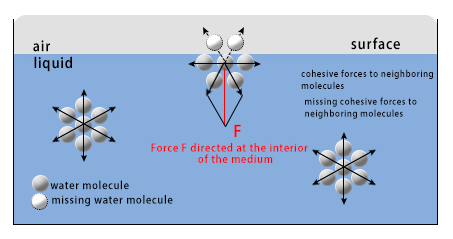
To understand the surface tension, consider water. Water is made up of a large number of molecules. These molecules pull each other with a cohesive force (A force is cohesive if it tries to bring two substances of the same kind together). Two kinds of cohesive forces—Hydrogen bond force and Van-Der Waal force exist between any two water molecules. If we consider a water molecule below the surface, it is uniformly pulled by water molecules all around it. So, the net force on the molecule is zero. For example, if a friend pulls you from your right and another pulls you from your left with an equal force, you do not move. The same principle applies to the water molecule below the surface.
(http://chemistry.tutorvista.com/physical-chemistry/surface-tension-experiments.html)
However, in the case of molecules at the water surface, the upward cohesive force is nonexistent (there are no molecules above them!). So, they experience a net pull downwards. (Note: the forces along the surface plane are still uniform and the resultant is still zero). Due to this reason, the surface molecules are constantly being pulled inwards. So, the area of the surface reduces unless the surface occupies the minimum possible area. In this condition, the molecules are so close that if pulled closer, they start to repel each other. Once this state has been reached, a considerable force is required to distort the surface. Hence, the water surface acts as a stretched membrane.
Thus we come to the conclusions that:
i. Water surface acts as stretched membrane so, certain insects can walk on it and steel pins float on the water surface.

(www.pixabay.com)
ii. For any given volume, the shape with the minimum surface area is a sphere. So, the raindrops are spherical.
Sources
i. Fluid Mechanics fundamentals and applications
ii. Fluid Mechanics
You answer all of my questions I got about this subject! Thanks :)
indeed
Thank you so much.
Thanks @gauss01 for your insights. I just knew that surface tension keeps insects floating but didn't know these deeper forces.
me too.
I am happy that you got some knowledge from my post.
A great introduction to surface chemistry, thanks :)
welcome. I am following your posts and they are awesome.
Keep it up. :D