With four new elements being named, the periodic table finally looks full. So it’s done, right? I mean, the periodic table is full. We’ve discovered all the elements up to 118, and filled in the whole bottom row.
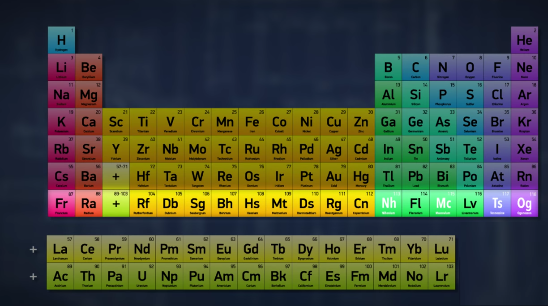
So there are no more elements to add, right?Well, not exactly. Just because the table looks full doesn’t mean that it’s complete -- it just means that we have to add another row.Or maybe more than one row, because no one’s really sure how many more elements can exist.
The first person to propose that you couldn’t just keep making bigger and bigger atoms was a chemist named Elliot Quincy Adams, who predicted in 1911 that no element could have an atomic weight larger than 256. Talking about elements in terms of their atomic weight might sound weird, since these days we count elements by their atomic number -- the number of protons in the nucleus. But back in 1911, protons hadn’t been discovered yet, so scientists organized elements by their atomic weights, instead. That’s the number of protons plus the number of neutrons. Back then, there wasn’t the single universal periodic table that we have today, either.
People used a bunch of different ones, and each table organized the elements in a slightly different way. Adams was proposing a new table, and he noticed that the last row of his table would end with an atomic weight of 256 -- the equivalent of an atomic number of 99 or 100.
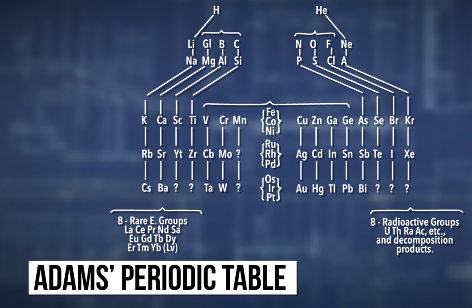
He figured that once his periodic table was full, that would be it: there could be no more elements after the one with a weight of 256. But … that wasn’t a very good argument. We made elements 99 and 100 more than sixty years ago, and we’ve made the next-higher eighteen elements since then.
Famous physicist Richard Feynman also predicted an end to the periodic table.

He calculated that an atom with 137 or more protons would violate special relativity. Atoms that big need electrons to help stabilize all that positive charge packed together in the nucleus. But more protons in the nucleus also means more force pulling the electrons in, so the electrons have to go faster and faster the bigger the nucleus gets.
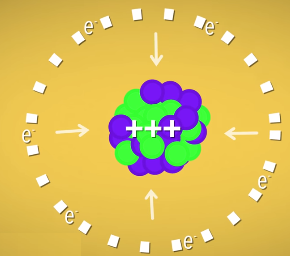
Feynman showed that in an atom with 137 protons, electrons would be moving faster than the speed of light. And going faster than the speed of light is impossible. So, Feynman said, there can’t be elements with atomic numbers higher than 137. He might have been wrong, though. His calculations made sense, but he was treating the nucleus like a single point at the center of the atom. That works fine for smaller nuclei, but it doesn’t work very well for the hulking masses we’re talking about here.
Physicists and chemists have even joked that element 137, once it’s finally created, should be named “Feynmanium” -- respectfully making fun of the Nobel Prize-winning physicist for one of the few things he was wrong about.
He probably would’ve liked that.
When Feynman’s calculation is repeated in a way that does account for the size of the nucleus, the number gets pushed all the way up to 173.
In atoms with more than 173 protons, electrons really do seem to need to go faster than the speed of light.So it’s possible that element 173 might actually be the end of the periodic table — though we don’t know for sure.
Elements tend to be more and more unstable as they go up in number, which means that, in general, it’s going to get harder and harder to make these elements as their atomic number increases.
All the new elements we’ve been making recently only exist for the tiniest fraction of a second before they decay into smaller atoms.
And maybe there’s going to be some point where we just can’t make them any more because they’re so unstable.
But physicists have also predicted what are known as “islands of stability” — sets of elements that are much more stable than you’d expect, because of the way their protons and neutrons are organized in the nucleus.
Instead of only lasting a tiny fraction of a second second, these elements might last days or maybe even years.
Some predictions say that the first island of stability might start right around number 122 or 126, depending who you ask and how they did the calculation.
And since we’ve already made all the elements up to 118, we’re pretty close! Which means that soon, we might be learning a whole lot more about islands of stability and how big our periodic table can really get.