Determination of platinum, palladium and rhodium in automotive catalytic converters using high-energy secondary target X-ray fluorescence spectrometry.Van Meel K, Smekens A, Behets M, Kazandjian P, Van Grieken R. Analytical Chemistry, 2007 Aug 15; 79(16): 63829.
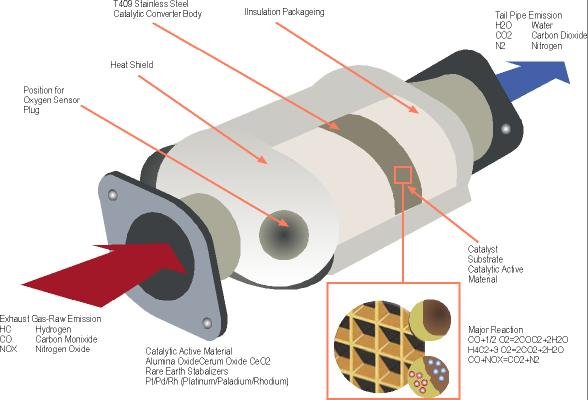
This paper discusses the use of X-ray fluorescence spectrometry to determine the precious metal composition of waste automotive catalytic converters (ACCs). Since 1981, vehicles in many countries have come equipped with three-way catalytic converters, so called because they catalyze three important pollution-control reactions. In the first, nitrogen oxides are reduced to nitrogen and oxygen by rhodium. Simultaneously, carbon monoxide and unburned hydrocarbons are both oxidized to carbon dioxide and water, by means of platinum and palladium catalysts. These metals are embedded in a honeycomb structure often made from the mineral cordierite. Due to the rarity and expense of the platinum-group elements (PGEs), and the environmental ramifications of their extraction, recycling and recovery of these materials is of particular environmental importance. Quick and precise analytical methods are needed in order to carry out such recovery activities profitably.
 Dean Kalma/ IAEA.flickr
Dean Kalma/ IAEA.flickr
Common methods to characterize PGE composition in ACCs suffer from various shortcomings. Inductively-coupled plasma mass spectrometry (ICP-MS) and optical emission spectrometry (ICP-OES) are most often used, but are quite time intensive, due to the many sample -preparation steps needed. Voltammetric methods present similar time-consuming digestion procedures.
The goal of the authors was to demonstrate a fast and direct method of PGE determination without losing the sensitivity commonly achieved with other methods. They aimed to measure concentrations with a relative accuracy of 1%, as compared with known samples measured using ICP-OES. Concentrations between ~0 and 500 ppm were analyzed fro rhodium, while concentrations were tested up to 2700 ppm for platinum and 7500 ppm for palladium. To achieve this, the authors used high-energy polarizing beam X-ray fluorescence (HE-P-XRF) to characterize PGEs in catalytic converters with fast sample preparation and high precision. The honeycomb matrix, known as the monolith, is ground into a powder and then pressed into a pellet using only a wax binder, and is then ready for analysis.
Joel Nelson. Wikimedia Commons
The authors reported the XRF technique to have an accuracy of 1% compared with ICP-OES, with a repeatablity better than 0.5%. The detection limits for Pt, Pd, and Rh were reported to be better than 5ppm when a 500 second irradiation time of secondary targets was used. The precision of the method was estimated to be ~1% as well, by analyzing 10 separate preparations of the same sample over a 10 day period. The average sample preparation time was found to be less than 2.5 hours after the catalyst is powdered. The measurement can take an additional 40 minutes. This is significantly faster than ICP-OES analysis; though the OES measurement takes only several minutes, preparation can take up to 5 days.
Thanks for great technical info, By the way I am bit illitreate but I try to read it and understand.
Thank you! This one is pretty technical for sure, I probably could have explained it better.
awesome work.i feel much better when catalyst will be eco-freindly