See Introduction here.
See Part 1 of Instrumentation here.
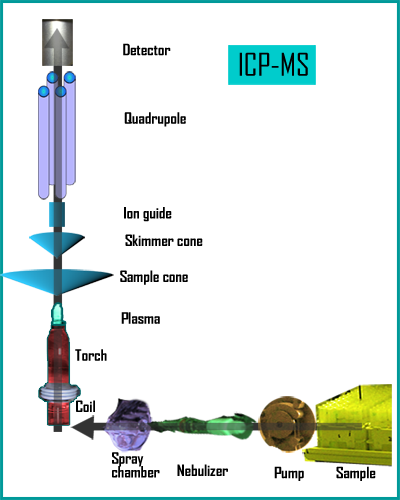
By passing argon through concentric quartz tubes with energy supplied at one end by an RF coil, a plasma is created that is around 6000°C, which causes analyte species to release electrons and form charged ions. The interface allows the plasma and the ion lens system to coexist at such a large temperature variance to ensure that ions pass into the mass spectrometer. This interface can consist of either two or three cones to focus the exiting beam and reduce pressure to the low levels needed for the quadrupole analyzer. With two cones, there is a two step pressure reduction which causes divergence of the beam, requiring further focusing. Most systems now use a third cone, called a hyperskimmer, which produces much less beam divergence and provides more ion transmission and greater stability. The cones themselves can be made of nickel, which is cheaper, or platinum, which offers longer life and slightly better focusing. If strong acids are to be used, the platinum cones are a better choice. For an ion to travel from the interface to the detector there should be no gas molecules getting in the way, so a vacuum system is required, including a combination of pumps. For reduced instrument maintenance, pumps that offer fluoropolymer lubrication are often chosen.
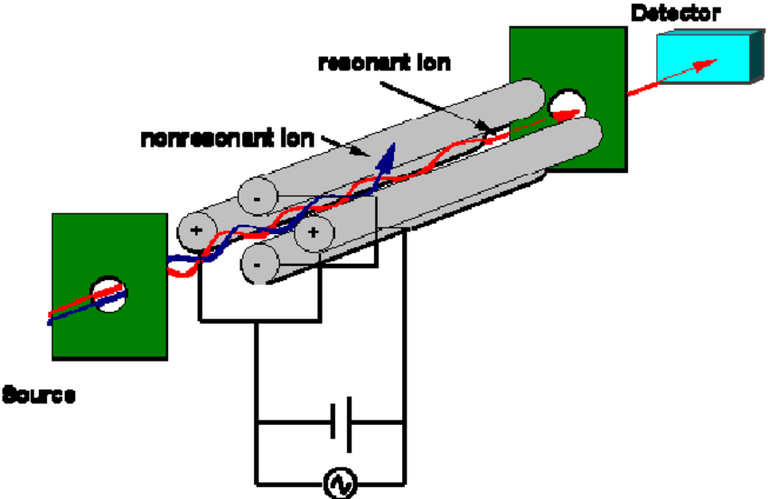
Quadrupole Analyzer.
Another important parameter is the collision/ reaction cell mode used. Collision cell mode is used when the interfering ions caused by the combination of argon and oxygen are larger than analyte ions, and thus lose more kinetic energy through more frequent interactions with the inert gas. An energy barrier at the exit of the cell can be adjusted so that only higher-energy analyte ions can pass, in a process called Kinetic Energy Discrimination. With this mode, the background signal is reduced, but so is the analyte signal. However this mode is easiest to optimize and use, and is often employed with high-variation environmental samples. In reaction cell mode, ions are passed through a cloud of reactive gas, such as ammonia. lnterferant ions are converted to a new species and lose their charge, thus dropping out of the system, while analyte ions are unaffected. This mode removes interferants more effectively, but requires the careful selection of reactive gas and can create new interfering species. For this mode, a quadrupole must be used as a mass bandpass filter, as hexapoles and octopoles will not perform correctly in this application. Reaction mode should be used for samples with more highly interfering species. If no interfering species are present, standard mode can be used.
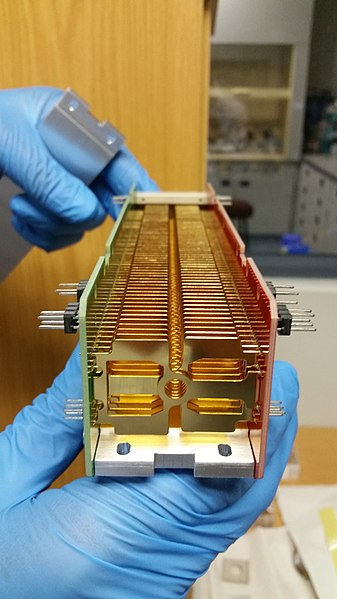
Collision Cell, by TheSteriodBiochemist. Wikimedia Commons
For the analysis of dietary supplements, which may contain ultra-trace and major components, it is useful to extend the upper range of the instrument, allowing for heavy metal determinations in the part-per-billion (ppb) at the same time as giving percent levels of major elements present. This can also be achieved if an octopole is used, by varying the electronic settings.
A final instrumental variable which must be selected is the type of analyzer used in the mass spectrometer. Most often used are quadrupole analyzers, which are small and easy to use but provide lower resolution for molecules with similar mass-to-charge ratios. Better resolution in these situations can be provided by a double focusing sector analyzer, though these are bigger and more costly.
@pinkspectre hello 👋 sir, Wow looks very nice. You read this post, you have made a very nice analysis, actually we do not have to steemit, actually many things are unknown to us, so thank you so much sir
With the rapid increase in the number of producers of dietary supplements, it's important to check the quality of stuffs in the consumers are digesting daily..
Thanks for much for this wonderful work.
Kudos to you.
This development is truly commendable @pinkspectre.. I would love to follow you @pinkspectre