TM0436 is a putative alcohol dehydrogenase enzyme, meaning its exact function and classification remain unknown. However, sequence, structure and genomic similarities between the protein and others of known function indicate its inclusion in the ADH class, likely in the L-threonine dehydrogenase subclass. The protein is homotetramer, consisting of four identical monomeric subunits of 368 residues.
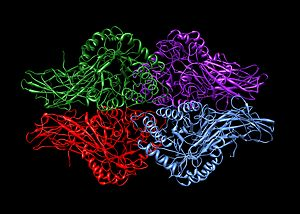
Homotetramer complex, Kt bug 87.Wikipedia
This protein binds the zinc ion Zn2+, and contains both structural catalytic zinc sites; one of each per subunit. Each unit also contains a Rossman fold, which facilitates the binding of NAD(H) ligands.
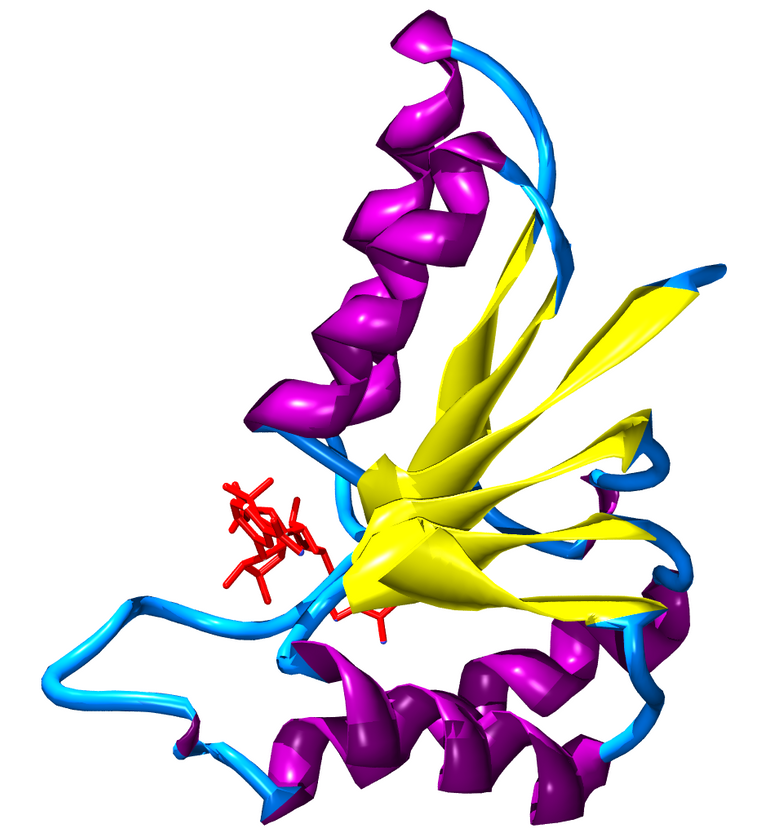
Rossman Fold.
TM0436, with an isoelectric point of 6.04 and a molecular weight of 40.5 kDA, is a member of the MDR protein superfamily, which contains alcohol dehydrogenases.
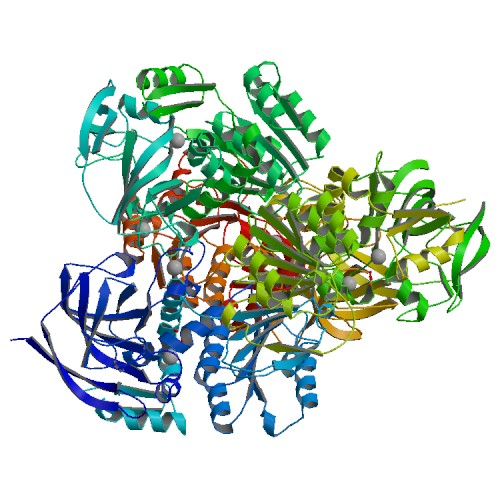
This image shows the crystal structure of TM0436 as determined by x-ray diffraction at 2.00 A resolution. By Joint Center for Structural Genomics rcsb.org
Alcohol dehydrogenase enzymes occur in many organisms, and catalyze the interconversion of alcohols with aldehydes or ketones. This is done using the energy derived from the reduction of NAD+ to NADH. This makes TM0436 an oxidoreductase - an enzyme that catalyzes the transfer of electrons from one molecule to another.
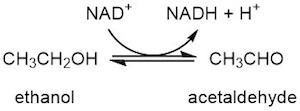
In many animals, these enzymes break down toxic alcohols , while in many bacteria and yeasts, ketones and aldehydes are converted to alcohols. Through this process of fermentation, a constant supply of NAD+, and thus metabolic energy, is ensured. Specifically, pyruvate from glycolysis is converted to acetaldehyde which is then reduced to ethanol by alcohol dehydrogenase. This process allows the continuation of glycolysis by the bacteria, but is also exploited by humans to produce alcoholic beverages from the yeast fermentation of fruits and grains.
thats right....nice post
Excellent post, friend, @pinkspectre

Your right friend.. I like this picture.
this brings back the memories