
"So, what's it gonna run on anyway?"
In this segment, I will be answering that question. You might've heard it runs on water, a simple and true statement, although it requires further explanation. And you might also be wondering where the fuels come from. Legit question, read ahead!
As we saw, the fusion process of the sun starts with 2 protons, who then release energy through a long and complex procedure in which a lot of other particles are involved. It would be unwise to replicate this process on earth because we found that there are way more efficient fusion reactions, reactions that only require things we already have here.
There are a lot of different candidates for nuclear fusion on earth, but the most promising one for the near future is deuterium-tritiumfusion (D-T fusion):
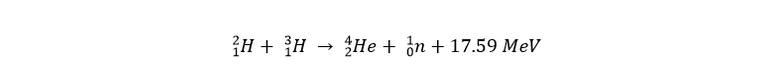
There are a lot of other fusion reactions that happen on different stars in our universe, but these either require way too extreme circumstances, or they’re just not efficient enough.
So why is D-T fusion so promising?
To start explaining this we first need to take a look at where the energy in a fusion reaction comes from. It all has to do with the fact that nuclei in a bound state have less mass than when it’s broken up into its components. As we saw with the measurements Aston, 4 separate hydrogen nuclei (protons) have more mass than 1 helium nucleus. This is a consequence of the binding energy of atoms – in the helium nucleus the particles are in a bound state – and this uses energy. The equivalence of mass and energy tells us that the mass that’s missing, also called the mass defect, is used as binding energy. All this results in a difference in mass before and after the reaction, and that mass defect is converted into energy.
Calculation of the binding energy of helium:

Helium has a relatively high binding energy (Figure 6):

This tells us that every fusion reaction in which helium is the reaction product is a very energy rich and efficient reaction, because the mass before the reaction is much higher than the mass after the reaction. There are a few atoms that form helium when they fuse, but deuterium and tritium are the most efficient.
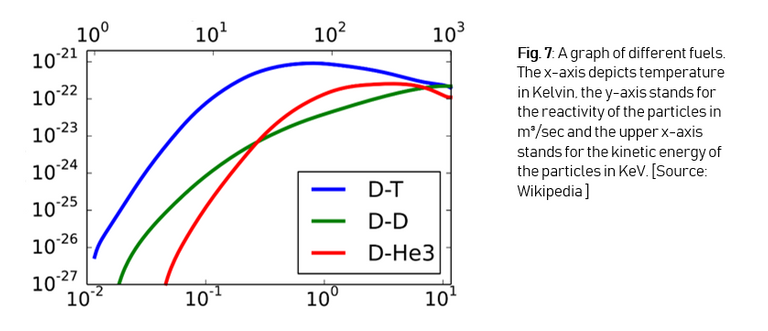
On the graph (Figure 7) we can see that D-T fusion has the highest reactivity with the lowest temperature (reactivity can be seen as the amount of particles of the D-T mix that undergo a fusion reaction each second), and that’s why deuterium-tritiumfusion is better than deuterium-deuteriumfusion or deuterium-helium-3-fusion, although both of these reactions have helium as the reaction product.
We can acquire both of these hydrogen isotopes relatively easy. Deuterium is a naturally occurring isotope, in about 6400 atoms of hydrogen you get from the ocean, there’s 1 deuterium-atom. Tritium is not naturally occurring, it’s a radioactive isotope with a half-life of about 12,3 years. The fact that tritium is radioactive, is one of the few disadvantages of deuterium-tritiumfusion. Tritium can be made by shooting a neutron on a lithium-atom:
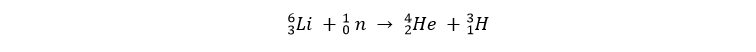
This process is called “tritium-breeding”. Lithium from land-based supplies is proven to last us at least another 1000 years. Further in this book we will learn that de neutrons that get released in the fusion reaction will be used to directly produce tritium in the reactor wall.
This is the end of post number 3 of this thread on nuclear fusion. The next post will be about how we're going to control the plasma. If you have any questions, feel free to drop them below. I will answer them! Thanks for reading.
With the help of heavy metals.