
Today’s post will present a concept which is rather abstract and somewhat complicated. However, keep reading! Learning about electron orbitals and their relationship to the periodic table will help you considerably in a variety of chemistry tasks. Perhaps most importantly, it can help you identify which elements react with which. So bear with me. I have separated this lesson in two parts and I promise that the next lessons will get easier! :)
As we saw in the previous post, electrons spin around the atom in electron "shells", depending on the energy state of each electron. Electrons with lower energy remain closer to the nucleus, because they are attracted by the protons’ positive charge. "Excited" electrons with extra energy, like to “jump” outwards.
Electron shells and subshells
The first, lowest energy electron shell, only fits two electrons. All consecutive shells fit eight electrons each. Electrons within these larger shells tend to follow different orbitals each, meaning that they don’t all just move round in circles; they tend to move in different paths to one another in order to satisfy space efficiency defined by the laws of attraction and repulsion. These orbitals form different shapes as different subshells.
Let’s take, for example, the element Neon. A neutral atom of Neon has a total of ten electrons. Two of these electrons fill in the first shell near the nucleus, in a round-shaped orbital called s-orbital. The remaining eight electrons fill in the second shell, a little further away from the nucleus. This second shell comprises of a larger round s-orbital and three dumbbell-shaped orbitals called p-orbitals (one left-right, one up-down, and one forward-backward), with two electrons each. We have now used up all of Neon’s electrons!
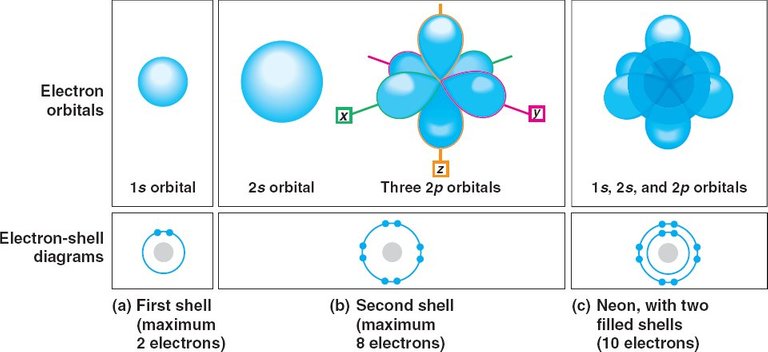 image credit
image credit
Orbital shapes
There are 4 main “types”of orbitals:
s, which is round-shaped
p, which is dumbbell-shaped
d, which is butterfly-shaped, and
f, which is crazy-shaped!
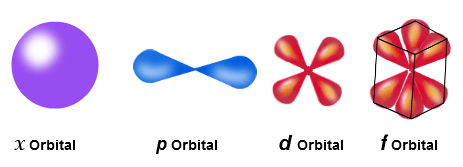
image credit
So what does all that have to do with the periodic table?
Well, electron shells and subshells are beautifully presented on the periodic table! Each horizontal row, a period, represents the number of electron shells an element has. For example hydrogen, the first element on the first period, has only one electron and therefore only one energy shell, an s-orbital. There it is on the periodic table, in the orange s-block!
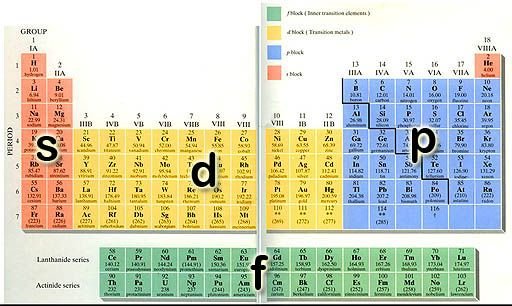
image credit
What about carbon? It’s in the second period and that means that carbon has two electron shells. Let’s check it out! A neutral carbon atom has six electrons. The first one of them will fit in the first energy shell in an s-orbital, just like hydrogen. The second one will also fit in the first energy shell in an s-orbital, just like helium. Since the first energy shell only fits two electrons, the remaining four electrons will have to move into the second energy shell. Two of them will move in a larger s-orbital, just like lithium and beryllium, and the other two in a dumbbell p-orbital, like boron and…carbon! Ending in a p-orbital, we can find carbon in the p-block.
We have to keep in mind that the second shell actually fits eight electrons, but carbon has only filled four. That means that these four are carbon's valence electrons, the ones that interact with other elements and cause bonds and reactions to happen!
Knowing about electron orbitals will help us identify the number of valence electrons for each element. Please don’t forget that the above methods are used to predict general trends and not the actions of individual electrons, as these are really unpredictable and random.
In the following lesson we'll learn how to identify how many valence electrons each element has by looking at the periodic table. Thank you for reading!

Your content has been chosen as the #3 featured on science-trail's weekly newsletter, congratulations!!
Join us on the science-trail Discord.
Thank you very much!
Hey, great article @elemenya
Follow me and look at some of my content, maybe even give me an upvote
https://steemit.com/@merrick
Thank you, @merrick ! I have followed you :)
Complicated stuff but surely helps the way you present it. Keep posting.
Thank you, @kyriacos .
you are welcome
This is a very good series of very nicely posts (that I have just discovered now)! Thanks for sharing that with the steemit community, and I am looking forward to read the rest!
Thank you so much. I try to do my best! :)
nice back to school breakdown
Thank you. I'm trying to break it down as simply as possible :)
This is a fantastic follow-upvote me