In my earlier post we discussed the EM waves and various different spectrums, like Visible Light, Ultra Violet Rays, X-Rays, Infra-Red Rays Etc. We know that the quantum particles have dual properties (Wave and particle) . the wave-like properties involve Diffraction pattern, interference pattern. the particle-like properties can be observed by observing phenomenon like collision, electric current and photo-electric effect.
The photoelectric effect is a phenomenon which introduces a powerful example in quantum mechanics for the wave-particle duality. Quantum Mechanism shakes the interpretations of classical Physics and pushes the boundaries to a whole new level.
Photoelectric effect Explained
Imagine a surface, on which an incident electromagnetic wave (not every electromagnetic wave, rather a high-frequency light wave). The high-frequency light waves are capable of breaking into the surface so that it can knock off the electrons from its orbit and induces the electric current. (The word photo means light hence the term photoelectrons means electrons induced by the incident light striking the surface).
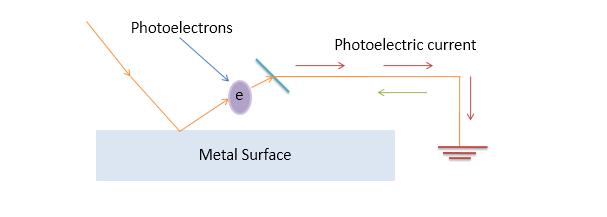
The metallic surface is connected to a circuit which takes the excited electrons into the ground. So the current starts flowing and this flowing current is called photoelectric current.
[Usage under creative commons Image Credit]
The Photoelectric emission starts when the radiation (visible light) start to falls on the metal surface.
We have discussed earlier in some cases the electromagnetic wave behave like a particle, which we can also be described by the phenomenon of the photoelectric effect.
We know that the light comes from the source in the form of packets, that means the energy is concentrated on the same place in the form of photons.
The photoelectric effect depends on the energy of incident light. Let's compare the effect induced by the different wavelength lights, let's say Red and Yellow. we know that the yellow light has more energy as compared to the red light.
(Note- If energy of incident light will high than the frequency will also be high)
E = hv
Here,
E = Energy
h = Plank's Constant And v = Frequency
Now if the red light (photons) incidents on the surface, no electron will emit as the energy provided by the red light is not enough to knock off the electrons in the outer shell of the metal atoms. And if incident yellow light (photons) than an electron will emit due to its high frequency. If we consider higher energy light (photons) of such as blue color then this will also emit the photoelectrons, we know that the yellow light has the minimum frequency required for the photoelectric effect to occur.
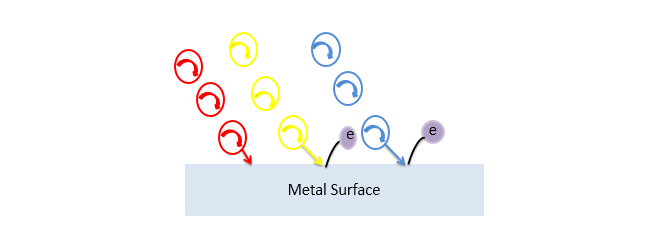
Threshold Frequency
For some metallic surfaces the blue light has the minimum energy and for some surfaces, the Ultraviolet rays have the minimum energy, so basically it depends on the surfaces that how much minimum energy is required to emit the photoelectrons. The minimum frequency required for the photoelectric effect is called the Threshold Frequency.
Now a new Question arrives in the mind that if the Red light photon cannot produce the electron from the surface (Because we know the red light does not have enough energy to produce electron), so why not introduce more Packets together to the surface so that it has enough energy.
work function
In the photoelectric effect, we require minimum energy for breaking the surface and emits the electron, this minimum energy is called work function that means the yellow light has the minimum energy which is equal to the work function.
If we consider a high energy light (like blue light) then it will not only break the surface and emits the electron but also emitted electron has some extra energy and this extra energy of the electron can be said kinetic energy.
The Kinetic energy of photoelectron depends on the frequency of the photon, it does not depend on the intensity of the light. If you need more photoelectrons then you will have to increase the frequency.
If we talk about the maximum kinetic energy then we will see the electron emits from near the surface has more energy as compared to the electron emitted from under the surface, because it has to spend some energy for traveling to the surface.
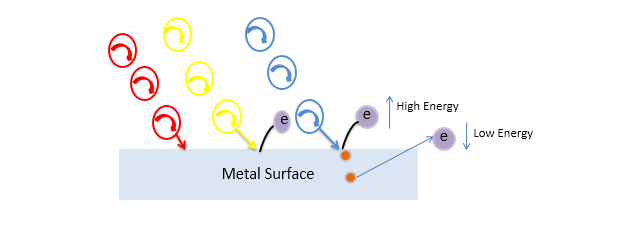
Hare we can say
Maximum Kinetic energy = hv - work function
The number of photoelectrons always depends on the intensity of the radiation, it does not depend on the frequency.
Applications
- The photoelectric effect is used in solar panels to create electricity using sunlight.
- This phenomenon is also used in creating various types of optical sensors, and we all know how important are those. ;)
| Citations | Further readings |
|---|---|
| 1. All images used in the post are used under CC0 and Sources for images are cited below the images unless they are self-created. | I would also recommend watching this video on youtube (Click here), |


gif Created by @foundation
Daily Fun Fact #100 -- "In 1921, Einstein was awarded the Nobel prize for his discovery of the law of the photoelectric effect"
More Information
Thanks for connecting a phenomena in physics to it's purpose in everyday objects. I'm very interested in these topics but only at the beginners 'pop-sci' level.
I'm following you :)
Thanks a lot, I appreciate that. :)
very informative post bro