Based on the principle that a combustible gas can be oxidized to produce heat, then using a Wheatstone bridge circuit, this change in temperature can be converted to an electrical signal.

A platinum coil embedded in a catalyst serves as the element where oxidation occurs, the reaction of the gas takes place at the surface of this element, in case of combustible gas the reaction is exothermic, heating up the catalyst element, causing a change in its electrical resistance and therefore unbalancing the bridge.
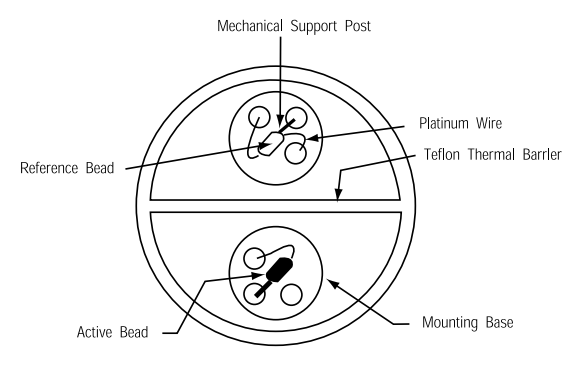
The catalytic sensor uses two beads, one active which oxidizes combustible gases, and one reference bead coated with glass that does not react with gases but rather with physical parameters as temperature, humidity and pressure. Concentration of gas present can be calculated from the resistance of the active bead compared to the “baseline” reference bead.
While other methods of detecting combustible gases are available, electrocatalytic sensors offer simplicity, accuracy and relatively low unit cost in a single-point detector.
References
General Monitors Staff, Fundamentals of Combustible Gas Detection