Searching YouTube on how to make opals at home gives you nothing worthwhile.
The only video coming close to my theory the creator creates a sol-gel substance and complains about not being able to get it to precipitate into a solid crystal.
This is one of the trade-secrets I don't think I'll be revealing, as well as one or two other things, but for the most part I will be describing the process of creating Gilson-grade synthetic opals, with no water content suitable for lampworking and high heat applications. I have researched and formed this theory in an effort to break a trade secret barrier and hopefully bring some prosperity to my life in the free market economy that I live in, but despite my intentions to profit off of my knowledge I believe the knowledge itself to be sacred and a thing to be preserved and not hoarded ultimately. So while I may not be the most vocal about this formula in real life, here I am more than willing to lay down the majority of the puzzle and also answer anyone he reads and has further questions and actually intends to use the knowledge as well, as long as I am able to respond.
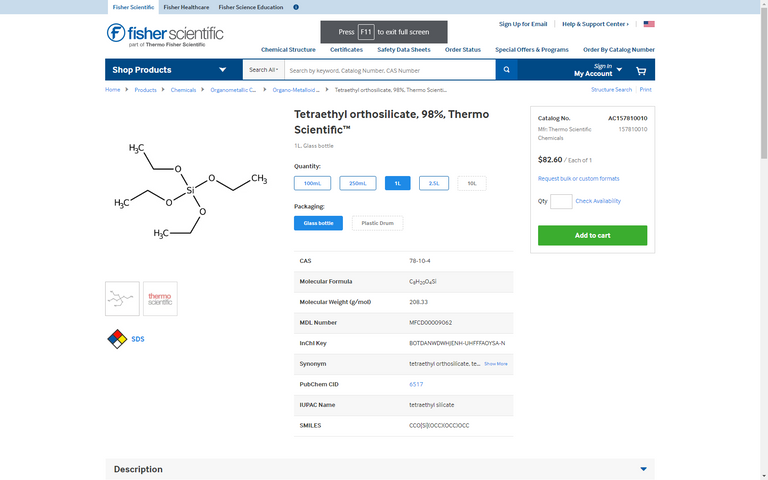
Our first topic is TEOS, or tetraethyl orthosilicate.
The first and most important step in this process is forming monodisperse Nano silica particles, or MNSP for short. This is the most important step because it builds the building block molecule needed for it to crystallize onto itself. This also happens to be the most delicate and to my understanding so far, difficult step of the process. That is because if the diameter of the MNSP isn't almost perfectly uniform the crystallization process won't occur. The diameter of the MNSP particles can be measured using Scanning Electron Microscopes (SEM), but if the profile of the sample varies more than 3% it will fail. Such uniformity can be achieved several different ways, and the way I intend on utilizing is through volumetric measurement of the other ingredients. The Tetraethyl orthosilicate is added to a solution of ethanol and kept on the higher end of average room temperature and at a constant stir for about 7 months. As I write this I'm currently having trouble finding the exact measurements, so worst case scenario I will run several batches with variable volumes and attempt the process of elimination.
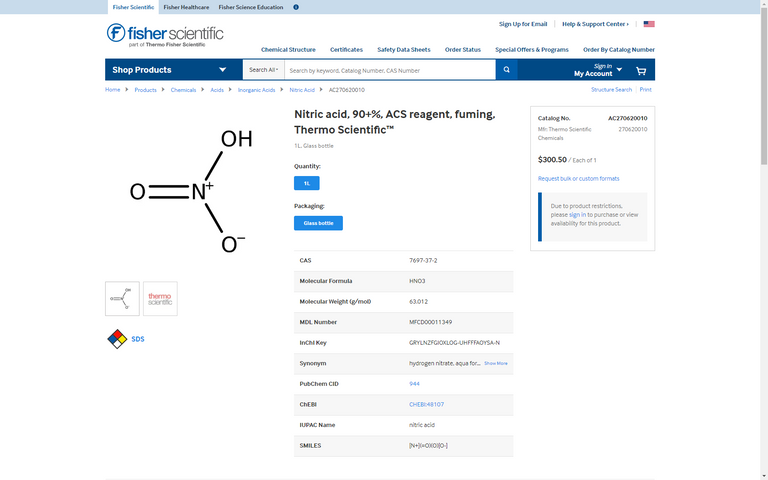
Nitric acid is also used in this mixture, as well as an inorganic salt
the specific one of which is determined by the desired end result color according to european patents, but there is also information detailing how the light refraction is controlled by the TEOS particle size.
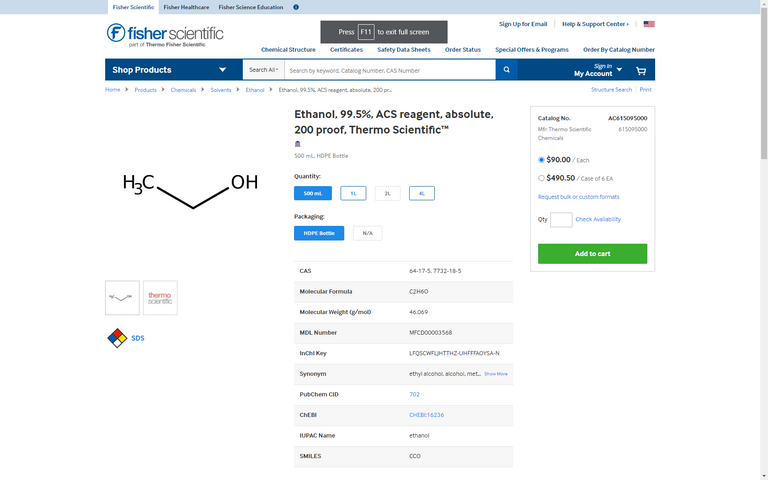
Ethanol is the main solvent used for the TEOS mixture.
After the MNSP are formed, and the nitric acid and inorganic salt are added, the solution should form a clear sol, which is then gelated over time at room temperature. This drying process occurs over 5-10 days in a dark place. Then the gel is heated in a lab furnace between 50-100 degress Celsius, then sintered slowly between 250 and 800 Celsius. This sintering process is one of the key steps that is often undiscovered by laymen I believe.
The resulting opal will have these characteristics -
a. hardness of 5.5 to 6.5 on Mho's scale;
b. refractive index of 1.37 to 1.47;
c. specific gravity of 1.96 to 2.20; and
d. a color from the group consisting of orange, pink, red, green, yellow and blue.



Congratulations @vagabond42069! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s):
Your next target is to reach 50 posts.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Check out the last post from @hivebuzz:
Support the HiveBuzz project. Vote for our proposal!
I'm curious if these monodisperse silica nano particles can be bought premade for easier oractice?
!hbit
!lolz
Success! You mined .9 HBIT & the user you replied to received .1 HBIT on your behalf. mine | wallet | market | tools | discord | subscribe | <>< daily