Greetings to everyone! Every day, as you go about your routine, you may not realize the hidden chemistry at work all around you. In the world of chemistry, one often-overlooked phenomenon has a profound impact on our daily lives—the colloidal state. Colloids are present in countless products and processes, from the food you eat to the medicines you take. Today, we'll take a closer look at colloids, explore their role in various aspects of life, and reveal how they quietly influence our world.
Colloids are enigmatic systems that consist of finely dispersed particles suspended in a continuous medium, such as a liquid, gas, or even a solid. These particles, which typically measure between 1 nanometer and 1 micrometer in size, are neither fully dissolved nor entirely settled. The colloidal state is more than just a scientific curiosity; it's a phenomenon with profound implications in various domains, from industry to biology.
The Colloidal State
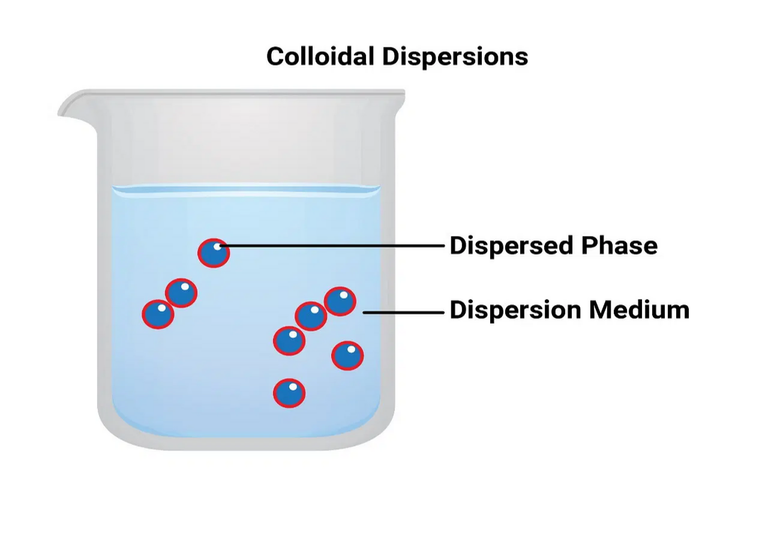
A colloidal system is a heterogeneous two-phase system consisting of dispersion of one phase, known as disperse phase into the other known as the dispersion medium. The dispersed particles usually ranges from 10-7 to 5 × 10-5 cm (10-5000 Å). In fact, the colloidal state is an intermediate state between a true solution and a coarse suspension. Depending on the physical state of the dispersed phase and the dispersion medium, it can be solid-liquid, solid-solid, liquid-solid, liquid-liquid, liquid-gas, etc. Generally, a colloidal solution or sol refers to the system in which the material is used as the dispersed phase and the liquid is used as the dispersion medium. Sols in water, alcohol, benzene etc. are known as hydrosol (aqua sols), alco sols, benzo sols etc. respectively.
Generally, two types of sols are recognized, namely lyophobic (hydrophobic) sols, which are solvent (water) hating and lyophilic (hydrophilic) sols which are solvent (water) loving. The first category includes weak sols, which can be easily precipitated after adding a small amount of electrolyte by heating or mixing, and the precipitated products cannot be returned to the colloidal state on addition of dispersion medium. This is why they are called irreversible sols or lefties. Examples of such sols are sols of metals, sulfur, sulfides and silver halides metal hydroxides etc. The stability of these sols is mainly due to the charge carried by the dispersed particles.
The second type of sol is one that is easily obtained by simply mixing the dispersed phase with the dispersion medium. These are not easy to precipitate, and if precipitation occurs, the separated particles only need to be mixed with a dispersion medium to return to the colloidal state. These are sometimes called as reversible sols. A lyophilic sol has a particle dispersion that has a great affinity towards the solvent and in fact that the colloidal particles in a lyophilic sol are highly solvated. The stability of lyophilic sols depends not only on the charge of the particles, but also on the extent of their solvation. Examples of this type are sols of proteins, gelatin, agar-agar, glue, gum arabic, starch in water etc.
The main difference between the two sols is the behavior of the electrolytes. Lyophobic sols are precipitated by small amounts of electrolyte, while lyophilic sols require a relatively high concentration of electrolyte for precipitation to occur.
Properties of Colloids:
Colloids exhibit a unique set of properties that make them stand out among other types of mixtures:
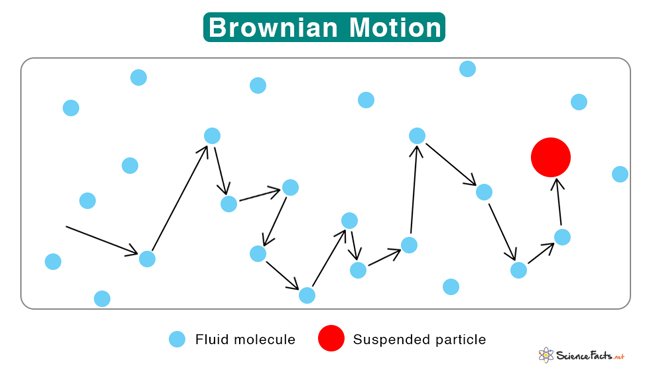
- Brownian Motion: Colloidal particles, driven by the incessant bombardment of surrounding molecules, engage in a never-ending dance known as Brownian motion. This perpetual, chaotic motion is visible under a microscope and played a crucial role in Albert Einstein's proof of the existence of atoms.
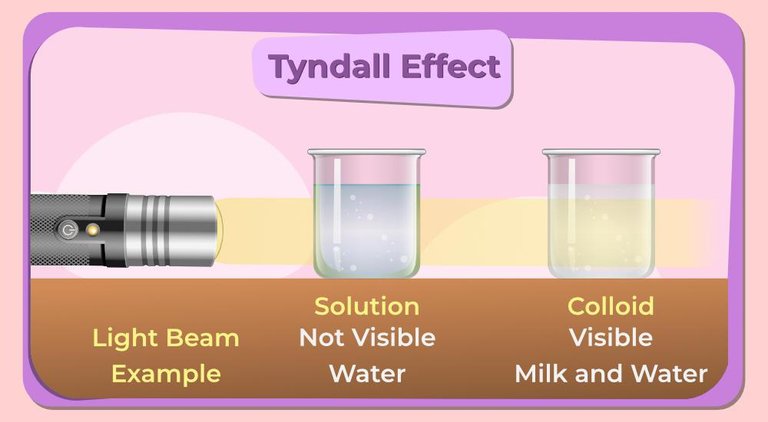
- Tyndall Effect: When a beam of light traverses a colloidal dispersion, it scatters, creating a visible path of light. This phenomenon, called the Tyndall effect, allows us to identify colloids and differentiates them from true solutions.
- High Surface Area: Colloidal particles boast an exceptionally large surface area in relation to their volume. This characteristic grants them outstanding reactivity, making them ideal candidates for various chemical processes.
These three chief properties of colloids make them valuable in various scientific, industrial, and practical applications, including food science, pharmaceuticals, materials science, and environmental remediation.
Precipitation of Colloids by Electrolytes
Although the traces of electrolytes are important for the stability of lyophobic sols, especially in water, small amounts of electrolyte often cause precipitation or coagulation of colloidal solutions. According to the Schulze-Hardy rule, for colloidal substances, ions carrying charges opposite to that of the colloidal particle is solely responsible to cause precipitation, and the precipitation ability of the effective ion depends on its valence. The higher the valence of the effective ion, the greater is the coagulating power.
An electrolyte's ability to precipitate is measured in terms of what is known as coagulating or flocculating value, which is defined as the minimum electrolyte concentration expressed in millimoles per liter of colloidal solution required for precipitation. In addition to the effective ion, this mainly depends on the nature of the precipitation process and the sol concentration. The smaller the coagulating value of the electrolyte, the greater the coagulating power.
According to one of the mechanisms of precipitation of colloidal solution by electrolytes, the effective ions are adsorbed, in preference to those carrying the same charge as the colloidal particle. Therefore, the electric charge of the particle is neutralized and a zero zeta potential is achieved. Consequently, the electrostatic repulsion of the colloidal particles ceases to act and aggregation occurs when the particles come in contact due to the Brownian motion.
Protection of Colloids
When to a hydrophobic sol a small amount of hydrophilic sol carrying the same charge or relatively large quantity of that carrying opposite charge is added, the hydrophobic sol becomes very much less sensitive to the precipitating action of electrolytes. In other words, the hydrophobic sol is protected by the hydrophilic sol from coagulating influences.
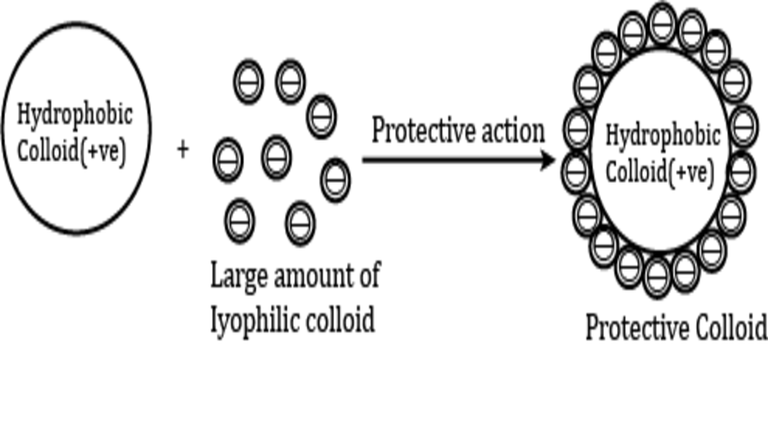
This phenomenon is called protection or stabilisation of hydrophobic sol, and hrdrophilic colloid used for achieving this protection is called a protective colloid.
The Unseen Influence of Colloids:
Food and Beverages: Colloids are culinary wizards. Have you ever enjoyed a creamy cup of coffee with milk? The fine coffee particles suspended in the liquid represent a colloid. Mayonnaise, ketchup, and salad dressings owe their texture and stability to colloidal systems. Even the froth on your beer is a colloidal formation of gas bubbles in a liquid medium. Colloids make your food more delicious and visually appealing.
Pharmaceuticals: In the world of medicine, colloids are vital. Drug formulations often use colloids to improve drug delivery. These systems control the release of medications, ensuring that they act effectively while minimizing side effects. Colloidal drug carriers are at the forefront of modern pharmaceutical research, making treatments more precise and efficient.

Cosmetics and Personal Care: Colloids also work their magic in the world of beauty. Creams, lotions, and shampoos often contain colloidal systems that enhance texture and the dispersion of active ingredients. They make these products more effective at moisturizing, nourishing, and rejuvenating the skin and hair.
Environmental Solution:
Colloids can be a possible solution to environmental problems. In wastewater treatment, they play an important role in removing impurities from the water by adsorbing pollutants to their surfaces. This process helps keep our waterways clean and safe.
Conclusive thoughts
The world of colloids is a silent but powerful force that touches nearly every aspect of our lives. From the food on your plate to the medications in your cabinet, colloids play a pivotal role in enhancing products and processes. As we become more aware of the influence of these microscopic particles, we can better appreciate the intricate chemistry at work in our everyday experiences. Colloids are not just a scientific curiosity; they are the unsung heroes of our modern world, making our lives easier, healthier, and more enjoyable. So the next time you sip a cup of coffee, apply moisturizer, or even treat a medical condition, take a moment to recognize the remarkable impact of colloids in your daily life.
Until we meet again :)

Classification of Colloids: Properties & Phases
Classification of dispersed systems and their general characteristics
Introduction to Colloid and Surface Chemistry. 4th. ed. Butterworth-Heinemann. Boston
The Complex Landscape of Opioid Analgesics: Addressing The Concerns |ChemFam #61|
Genetic Engineering: Pioneering Progress or Ethical Predicament? |ChemFam #60|
The Guardians Against Microbial Menace: Antibacterial Agents |ChemFam #59|
The Cholesterol Conundrum: The Story of Statins |ChemFam #58|
Unveiling The Control Of Chemistry: How Hormones Dictate Our Mood |ChemFam #57|
Thermodynamic Versus Kinetic Control of Reactions |ChemFam #56|
Bosons: The Quantum Glue That Holds The Universe Together |ChemFam #55|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery-II |ChemFam #54|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery |ChemFam #53|
Helium: The First Noble Gas |ChemFam #52|
Hydrogen: The Simplest Atom |ChemFam #51|
Elements, Atoms and Atomic Theory |ChemFam #50|
Have You Thanked A Clod Today? |ChemFam #49|
Nuclear Energy: Will It Rise Again? |ChemFam #48|
Soaps: An Essential and Effective Cleansing Agent |ChemFam #47|
Chemicals in Food : Debunking Myths and Ensuring Safe Consumptions |ChemFam #46|
Unveiling The Secrets of Antiseptics and Disinfectants |ChemFam #45|
What are Antimicrobials and Antimicrobial Drugs? |ChemFam #44|
Therapeutic Action of Different Classes of Drugs |ChemFam #43|
PS The thumbnail image is being created by me using canva.com


Those types of colloid feel so hard to pronounce. Reading them was more like me biting myself in my mind. Thank you for the cool work on colloids.
!PIZZA
$PIZZA slices delivered:
@balvinder294(5/5) tipped @splash-of-angs63
This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
Congratulations your publication has been chosen among the best of the day.
KEEP CREATING GOOD CONTENT.