Theory of electrolysis
In the early 1800s, the scientist Faraday first demonstrated that electricity flows through a solution through the movement of charged particles. It is called a wanderer in Greek because it can move in a charged particle solution. It should be noted here that before this, people had no idea about ions. In 1834, Faraday named the first positively charged particle cation and the negatively charged particle anion. Time goes to the catheter cathode and completes the deionization reaction by receiving electrons and through that, the charge is released. On the other hand, we will go to the anion anode and leave the electron to complete the oxidation reaction and its become charge free. Another scientist later suggested that every electrolytic substance contains both active and inactive molecules. When the active molecules dissolve in the solution, they dissociate into ions and participate in the conduction of electricity. Inactive molecules, on the other hand, do not change in the solution. Degree of dissociation says. An electrolyte that has a high dissipation value is called acute electrolysis and electrolysis that has a relatively low dissipation value is called mild electrolysis.
Dispersion level, alpha =
Number of dissociated molecules / Number of matte moleculesElectrons enter the solution through the cathode (negative electrode).
Electrons leave the solution with an anode (positive electric current).
Explanation of electrolysis
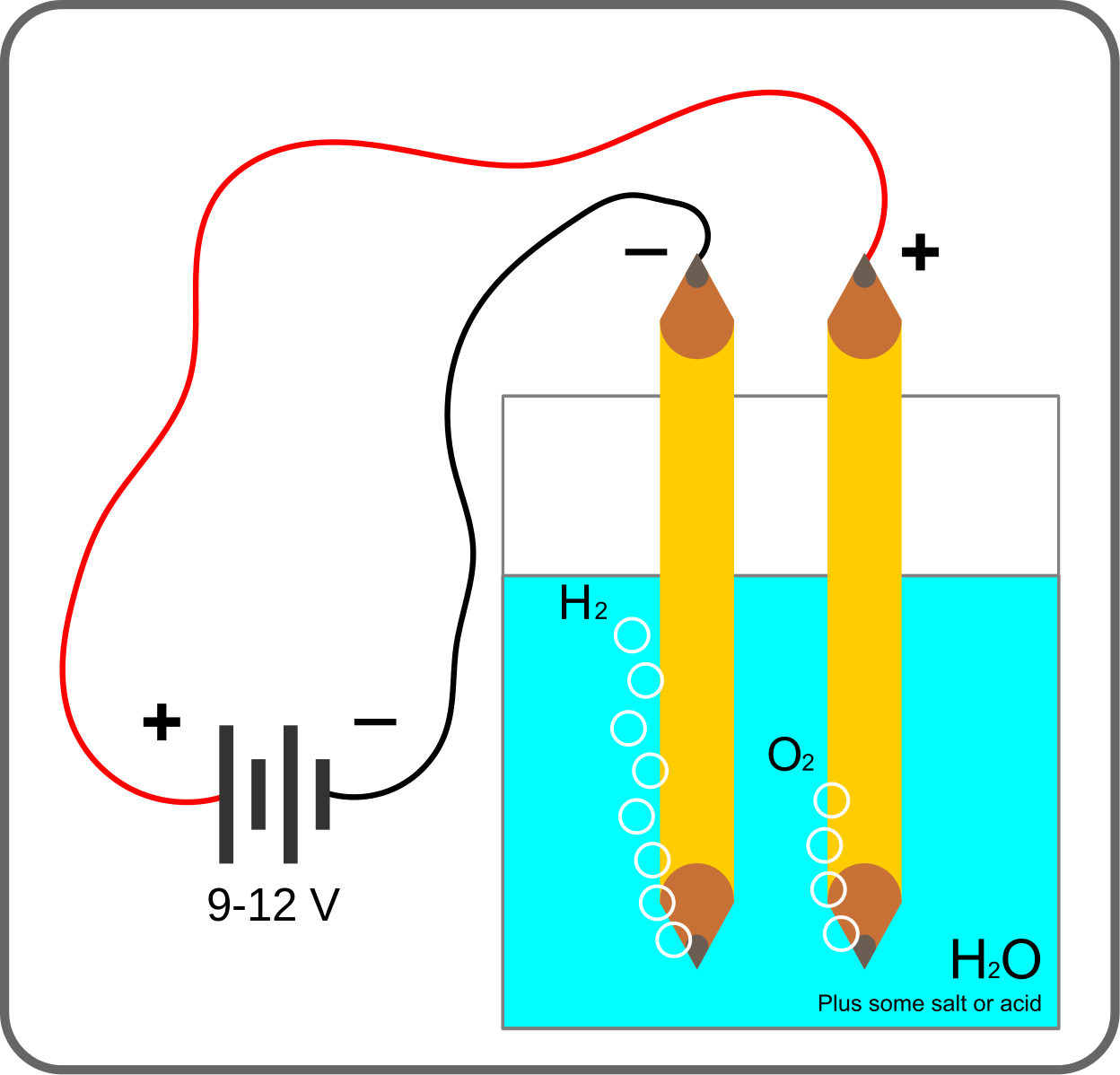
When electrolysis of molten salt is done, the metal ion (cation) is attracted to the cathode. Metal ions actually provide electrons with a negatively charged electrode near the catheter. The resulting metallic ions.
Cation) is deposited in the catheter.
- Na * (melted) + e "→ Na or (Cu2 +) + 2e → Cu
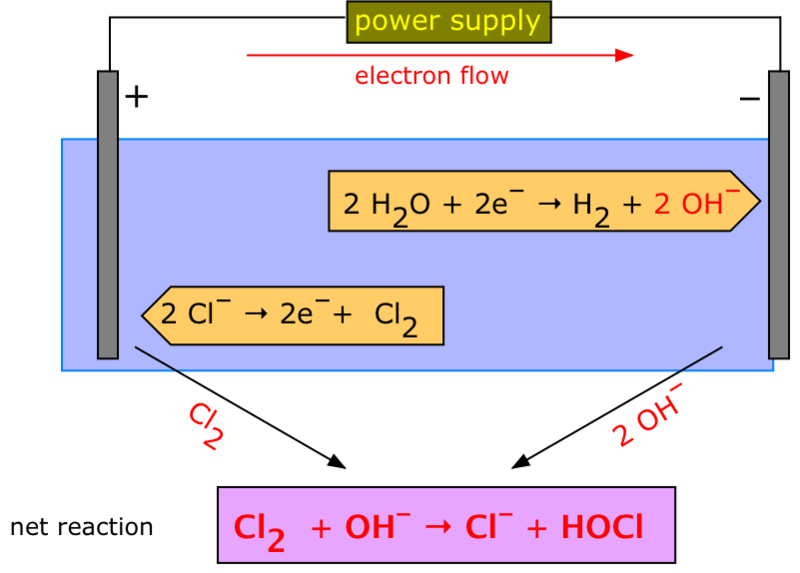
On the other hand, negative ions (anions) are attracted to anodes. Negative ions come to the anode . Electrons are oxidized by leaving.
- (2CI-) (molten) → Cl2 (gas) + 2e
here receives electrons from anode anion. These electrons go to the cathode with the help of the exterior and dissociate the cation. If an aqueous solution of salt is used without the use of molten salt in electrolysis, sometimes the yield is not as expected. For example, in the electrolysis of a light aqueous solution of sodium nitrate, sodium ions are in the cathode. Instead, Parton is poisoned. This is because, in this case, some part of the water is ionized by proton and hydroxyl ions
Provides.
2H2O) → H2O * (aq) + OH- (aq)
H3O (aq) + e → 1/2 H2 (g) + H2O
Thus, both nitrate and hydroxyl ions are present around the anode. Since hydroxyl ions are more easily oxidized than nitrate ions, nitrate ions remain in the solution and hydroxyl ions oxidize to produce oxygen.
- (4OH-) (aq) -4e → O2 (g) + 2H2O (l)
Thus water is analyzed when electrical energy is applied to a solution of NaNO3, . Of other solutions similar questions may arise in the case. For example, if Cu++ and Zh++ ions are together in a solution, then Zn+- is in electrolysis. Cu++ is dissolved, Zn++ remains in the solution. If a solution contains bromide (Br -) and iodide (I-) ions
Then the iodide ions are oxidized and the bromide ions remain in the solution. The main reason for this is the difference in electric gate voltage. And this can be explained by the electrochemical arrays
series). It is explained below. The anion participates in the oxidation reaction by releasing electrons in the anode. The catheter receives cation electrons.
Reference : Chemistry- Electrolysia