Electrolysis is an amazing process that is gaining momentum in both social and industrial fields. There is a role for electrolysis in various everyday tasks. We use various metal objects as gifts in various activities of social life, entertaining guests and also in social occasions. To enhance the beauty and durability of such items, a thin coating of precious metal is applied on the relatively less expensive metal. At present, due to some security issues, it is not always possible to get out of expensive jewelry.
So the use of artificial jewelry as an alternative to expensive jewelry has increased tremendously, such jewelry is called imitation jewelry. Artificial jewelry is similarly made with a small amount of precious metal coating on relatively less expensive metals. That is all these things used in our social life are made with the help of electrolysis. Electrolysis is also widely used in the extraction of metals and in the production of various chemicals.
Here are some examples of electrolysis process used in industry
Extraction of sodium metal from salt of molten sodium chloride by electrolysis method (down method).
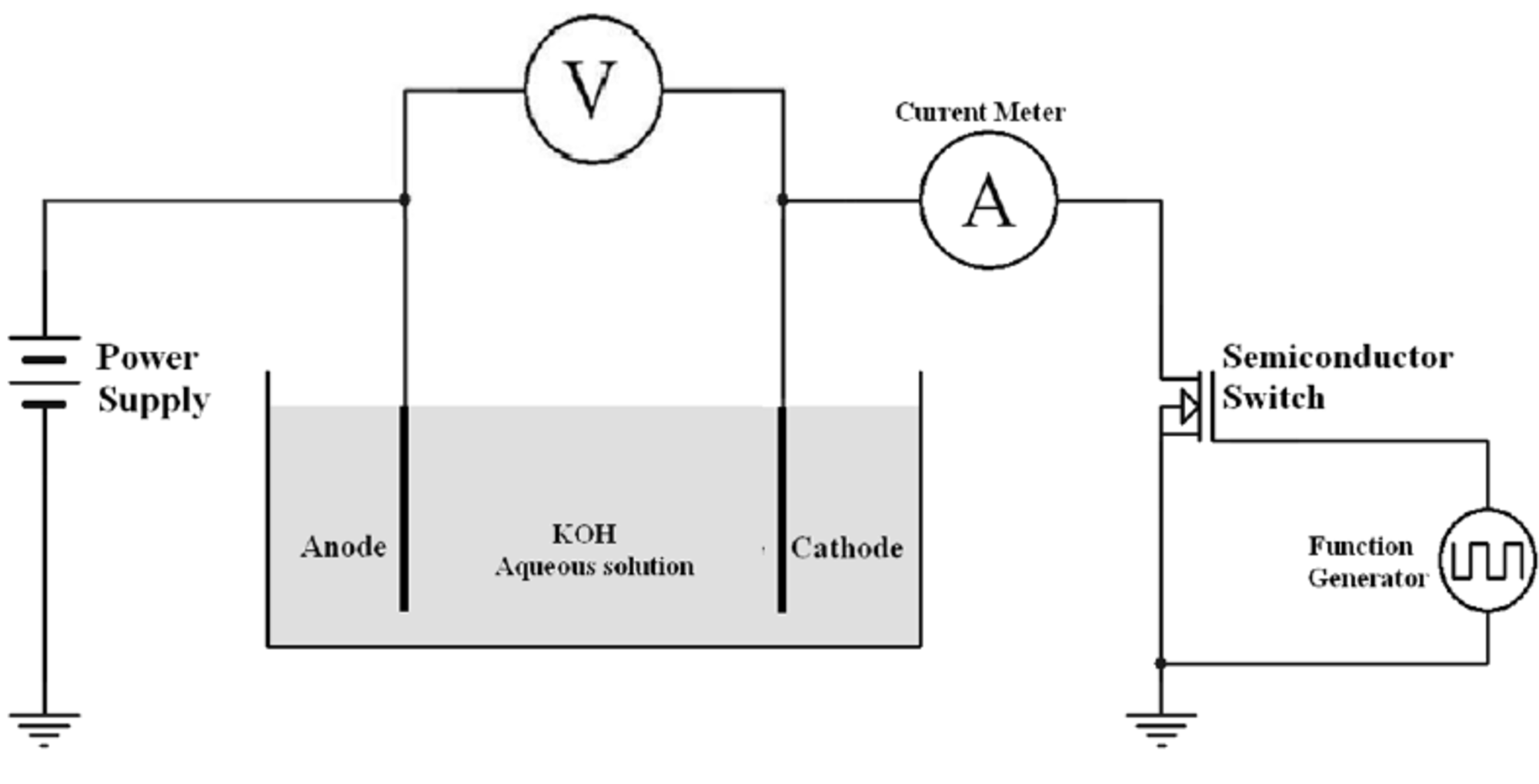
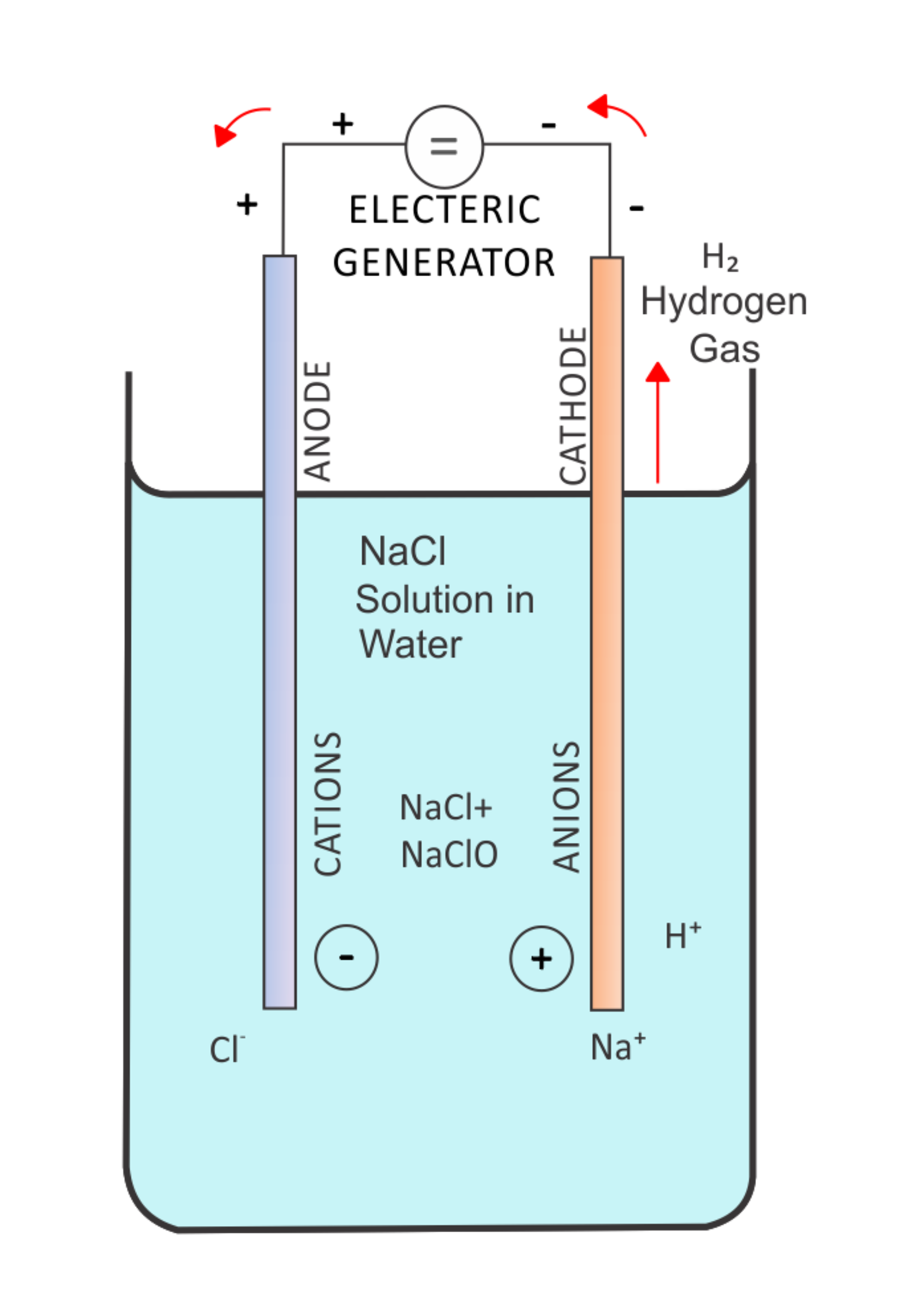
Method of production of sodium hydroxide by electrolysis from aqueous solution of sodium chloride in mercury cathode cells.
Method of production of sodium chlorate by electrolysis of aqueous sodium chloride solution.
The process of preparation of chlorine from the salt of sodium chloride with the help of methods 1 and 2.
Method 2 Production of hydrogen gas from aqueous solution of sodium chloride.
With the help of electrolysis from molten calcium and magnesium chloride, magnesium and
The process of calcium extraction.Aluminum extraction process from bauxite by electrolysis.
Anodic oxidation of aluminum and dyeing of aluminum metal.
The process of refining copper with the help of electrolysis using unrefined copper as an anode.
Applying electroplating especially nickel or chromium coating.
Reference : Chemistry- Electrolysia

Congratulations @rana007! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s):
Your next target is to reach 800 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out the last post from @hivebuzz:
Support the HiveBuzz project. Vote for our proposal!