Linus Pauling.
Yes, that's what the only person in history to win two unshared Nobel Prizes looks like.
Source: Wikimedia Commons contributors
Introduction
Ever have one of those days where you wonder at how people can perform so much better than you, with seemingly much less effort? There you are, working your fanny off, sleeping 4 hours a day to make a deadline, and in waltzes an employee who barely ever shows up at work, hands your boss a prize-worthy assignment, thereby stealing your job promotion — ever had that? And as a result you feel less of a person, and you wonder if you were ever really cut out for this, if maybe you should quit, if maybe your boyfriend's mom is right and you're really not good enough for him?
Well, I'm about to make those days of yours even worse! Because this is going to be a post about how Linus Pauling made one of the most significant discoveries in chemistry and biology while he was sick in bed. Bedridden, but restless, he thought, "I got paper, so why not make some origami and try to figure out how proteins are folded?"
And figure out he did. The guy's spatial IQ must've been through the roof!
So let's begin!
A detour
See that "secondary" in the post title? Secondary comes after primary and before tertiary. To put Pauling's discovery into context, if not fully appreciate it, we'll be well-advised to hew close to the semantic order line and do the 1 before the 2. So let's start with proteins' primary structure.
Building blocks and primary structures
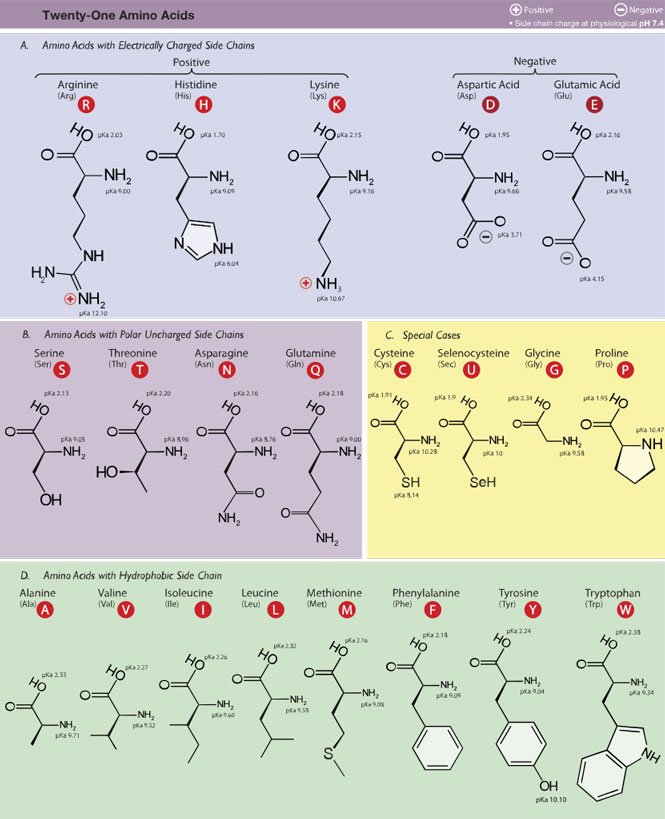
The primary building block of proteins is the amino acid. For the purposes of an intro-level biology post, there's 20 of them. Here they are, the 20 amino acids:
Source: Wikimedia Commons contributors
So these are the twent—wait a minute! If I remember my kindergarten math, I here count 21, not 20! Someone smuggled herself in!
Investigating the chart, I see a selenocysteine amino acid that wasn't there when I was memorizing my amino acids. I'm either too old, and things have happened in the meantime, or something much more sinister is going on.
Turns out, selenocysteine is not universal to all life. Its inclusion in the group of 20 is therefore controversial. Read more about it here (I recommend clicking on 'PDF' for better viewing) and decide for yourself.
You'll also notice that the amino acids have an identical part:
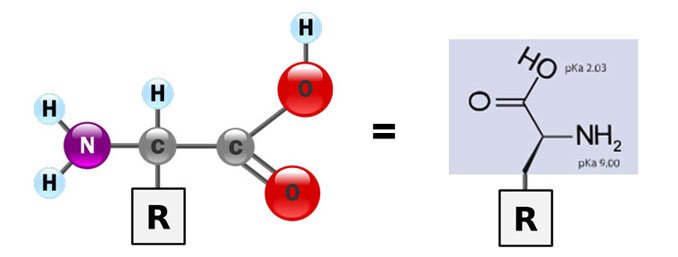
Source: Wikimedia Commons contributors 1 & 2, modified.
That part is identical to all amino acids. The thing that gives each amino acid their different identity, is the R, which is called the side chain. There can be 20 (or 21!) amino acid side chains, which means there can be 20 different amino acids.
These amino acids can link together by a process called dehydration synthesis [2]. Dehydration cos you take out water, and synthesis cos you create a (peptide) bond between the molecules when you take out the H2O.
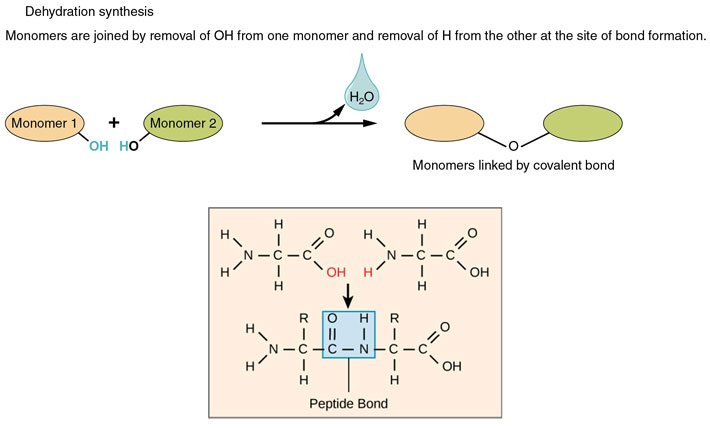
Source: Wikimedia Commons contributors a & b, modified.
The primary structure of a protein is simply this: amino acid connected to amino acid connected to amino acid...and so on. The number, order, and identities of these amino acids make up the primary structure of a protein.
The huge diversity of proteins
Suppose I want to stick two amino acids together, in what's called a dipeptide. How many dipeptides can I make? I have 20 options for the first amino acid, and 20 for the second. I can therefore make a whopping 400 dipeptides just out of 2 amino acids!
What about tripeptides? 8000. Tetrapeptides? 160,000. Now note that these aren't yet proteins! What's the smallest number of amino acids you need to make a protein? I don't know, but a paper suggests it might be 10. That's 10,240,000,000,000 different possible proteins, with just half the number of amino acids we got at our disposal. You can see why some biologists once thought proteins were the most likely seat of inheritance, and scoffed at chromosomes! [6: DNA or protein?]
Protein secondary structure
You can also see why predicting the secondary structure of a protein is difficult. How are all those amino acids going to arrange themselves? Are the hydrophilics going to partner up with other hydrophilics, pushing the hydrophobics into a group of their own, like we saw in the last post? What about all these new charged amino acids?
This is quite tricky business, and a whole field has sprung up trying to solve the so-called protein folding problem [7]. If you ever find yourself sick in bed with nothing to do, there's plenty of unsolved problems in biology, that you can find toward the end of this article here, that you can try your hand at. There's also a TEDx talk on the protein-folding subject and its importance.
Now note, the protein folding problem is our problem, not the proteins'! The proteins fold themselves up pretty fine and easy thank you very much. [4: Driving force of protein folding] All you gotta do is throw them into some cell cytoplasm and then just shake shake shake and they just rapidly fold themselves up.
And you don't even need to do the shaking part, I just made that up.
But just because you can't solve the whole protein folding problem in one go, doesn't mean you can't make significant inroads.
Enter Linus Pauling
In 1948, Linus Pauling caught what is probably the most productive cold in the history of science.
Here's how it went down.
He caught a cold. (You should watch the video.)
And so there he was, bedridden. It's 1948, so no facebook, no wifi. So what do you do? Obviously you do some armchair sciencing. So that's what he did.
He took some sheets of paper and started folding them.
He simplified. He ignored the amino acid side chains. He concentrated on the possible hydrogen bonds that could be made by the backbone. [8]
So he came up with a helical structure. (Anyone familiar with the double helix structure of DNA, will immediately realize how important this discovery, or brainstorm, will prove in the future.)
He called it the alpha helix.
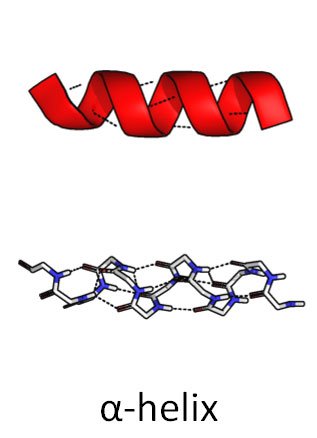
Source: Wikimedia Commons contributors, modified.
Dotted lines represent hydrogen bonds.
But the cold wasn't done with him yet
When Linus was finished with the alpha helices, he checked his temperature, and he saw he still had a cold. So he said, "ah, what the hell, I'll do one more". (The historicity of the phrasing may be inaccurate.)
So he came up with another way he could imagine the hydrogens bonding to each other: the beta sheet.
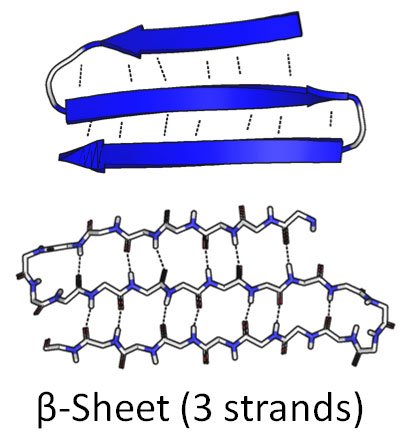
Source: Wikimedia Commons contributors, modified.
Dotted lines represent hydrogen bonds.
Outcome
We now know alpha helices and beta sheets form the backbones of tens of thousands of proteins [9]. Plus, Pauling's discoveries played a large role in establishing the discipline now known as molecular biology. [11]
So this was some good origami work! So good, in fact, that biology classrooms have their students replicate it. [10: Folding Exercise]
Yeah, I know: poor trees.
Armchair science
What I love about this historical tidbit, is that it showcases how discoveries can often be made just by thinking about things. As much as I like feet-on-the-ground science, I have the kind of brain that finds theory much easier to grasp and innovate in. Might be part of the reason why I went into philosophy.
Often people find it difficult to understand what the philosopher does, because they have a hard time imagining how — or even believing that — discoveries can be made just by thinking hard at stuff.
Yet this is hardly something only philosophers do! Every science has its theoretical side: theoretical physics, theoretical biology, etc. What's more, the most famous scientists are often those who work only with pen and paper (and, I assume, an armchair, or at least a chair), far outshining in the public eye those who do the data extraction and the manual work, fair or not.
Curtain close
If I have succeeded in my goal, this article will have made you feel a little bit more insecure, less comfortable in your skin, and more doubtful of your abilities. I hope, at the very least, in the soon-to-be-upon-us winter, you will be comparing yourself to Pauling, and constantly asking yourself, "how productive will my cold be this season?" :P
References:
Robert Longtin; A Forgotten Debate: Is Selenocysteine the 21st Amino Acid?, JNCI: Journal of the National Cancer Institute, Volume 96, Issue 7, 7 April 2004, Pages 504–505, https://doi.org/10.1093/jnci/96.7.504
Wikipedia contributors, "Dehydration reaction," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Dehydration_reaction&oldid=802386593 (accessed November 17, 2017).
What is the minimum number of letters required to fold a protein? Ke Fan, Wei Wang; J Mol Biol. 2003 May 9; 328(4): 921–926. https://www.ncbi.nlm.nih.gov/pubmed/12729764
Wikipedia contributors, "Protein folding," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Protein_folding&oldid=807411260 (accessed November 17, 2017).
Wikipedia contributors, "Linus Pauling," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Linus_Pauling&oldid=810436895 (accessed November 17, 2017).
DNA or protein? Nathan H Lents, Ph.D. “DNA I” Visionlearning Vol. BIO (2), 2008. https://www.visionlearning.com/en/library/Biology/2/DNA-I/149
The Protein-Folding Problem, 50 Years On; By Ken A. Dill, Justin L. MacCallum; Science23 Nov 2012 : 1042-1046. https://www.ncbi.nlm.nih.gov/pubmed/23180855
"The DNA Story." 1973. Created by VSM Productions. Produced by Ronald Fouracre and Peter Shaw. Distributed by John Wiley & Sons, London, New York. http://scarc.library.oregonstate.edu/coll/pauling/proteins/video/1973v.3-alpha.html
Eisenberg, David. “The Discovery of the Α-Helix and Β-Sheet, the Principal Structural Features of Proteins.” Proceedings of the National Academy of Sciences of the United States of America 100.20 (2003): 11207–11210. PMC. Web. 17 Nov. 2017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC208735/
Pikaart, M. (2011), The turn of the screw: An exercise in protein secondary structure. Biochem. Mol. Biol. Educ., 39: 221–225. doi:10.1002/bmb.20487 http://onlinelibrary.wiley.com/doi/10.1002/bmb.20487/full
Francis Crick, The Impact of Linus Pauling on Molecular Biology, Special Collections, Oregon State University Libraries, Corvallis, OR 97331-4501 http://oregonstate.edu/dept/Special_Collections/subpages/ahp/1995symposium/crick.html
steemSTEM is the go-to place for science on Steemit. Check it out at @steemstem or browse the #steemSTEM tag or chat live at steemit.chat
The famed Watson and Crick of DNA structure asked Linus Pauling many questions about his structure work in proteins, but never returned his call to them. Apparently, there is a loophole in scientific information exchange custom, in which if queries only flow one way, the receipient does not need to provide credit for the answers received. Pauling, himself, may have been thinking about DNA at the time, and the famous duo did not want to share credit.
Pauling's elucidation of Vitamin C molecule made nuclear submarine warfare possible. Without Vitamin C manufacture, the nuclear powers would be limited only to surface traveling vessels in land and air, as the scourge of the navy, scurvy, would have made long-term naval vessel operation impossible.
Oh in my book it's Pauling who discovered the structure of DNA!! He had the helix down and everything, he just had it inside out. A silly mistake, really, and it would've taken him a very short time to realize it, which is why Watson and Crick rushed to the presses (Nature magazine) like crazy, writing up "their" discovery in a single hurried page.
@OriginalWorks
The @OriginalWorks bot has determined this post by @alexander.alexis to be original material and upvoted(1.5%) it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!